J.ophthalmol.(Ukraine).2020;5:13-20.
|
http://doi.org/10.31288/oftalmolzh202051320 Received: 22 June 2020; Published on-line: 27 October 2020
Levels of pro-inflammatory (TNF-α and IL -6) and anti-inflammatory (IL -10 and IL- 4) interleukins in ocular herpes patients with frequent and infrequent recurrences N. I. Khramenko, T. B. Gaidamaka, G. I. Drozhzhyna, L. N. Velychko SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) E-mail: khramenkon@gmail.com TO CITE THIS ARTICLE:Khramenko NI, Gaidamaka TB, Drozhzhyna GI, Velychko LN. Levels of pro-inflammatory (TNF-α and IL -6) and anti-inflammatory (IL -10 and IL- 4) interleukins in ocular herpes patients with frequent and infrequent recurrences. J.ophthalmol.(Ukraine).2020;5:13-20. http://doi.org/10.31288/oftalmolzh202051320 Background: Herpes simplex virus ocular infection is the major cause of corneal blindness in developed countries. The methods used for the treatment of recurrent herpes infections give only temporary remission, and the results of studies on prevention and prediction of the course of the disease are contradictory, which makes management of these infections not only medically, but also socially important. The mechanism of recurrence of herpetic stromal keratitis (HSK) infection has not been clearly understood, since the disease involves complex pathophysiological mechanisms and immune factors (including cytokines) are complexly interrelated in the pathogenesis. Purpose: To assess serum levels of proinflammatory cytokines (TNF-α and IL-6) and anti-inflammatory cytokines (IL-10 and IL-4) in ocular HSK patients with frequent and infrequent recurrences during remission and recurrence. Material and Methods: Thirty-three in-patients (15 patients and 18 patients experiencing less than and more than one recurrence annually, respectively) treated for recurrent HSK at the Department of Corneal Disorders of the Filatov Institute were included in the study. Serum IL-4, IL-6, IL-10 and TNF-α levels were determined using enzyme-linked immunosorbent assay (ELISA) kits from Vektor-Best (Novosibirsk, Russia) according to the manufacturer’s instruction. Photometric measurements were performed on an ELISA plate reader (Stat Fax 2100, Awareness Technologies Inc, Palm City, FL). Reference normal values were taken from the manufacturer’s instructions. Results: We found that the mean serum TNF-α level in patients with infrequent recurrences and in those with frequent recurrences during remission was 2.6 times and 4 times, respectively, higher, and during recurrence, 5.6 times higher, compared to normal values, which is likely to indicate a subclinical inflammatory process. Both in patients with infrequent recurrences and in those with frequent recurrences of keratitis, the mean serum TNF-α level was 50% higher during recurrence than during remission. The mean serum IL-6 level during remission was comparable to, and during recurrence was 3 times higher than the norm, with no significant difference between patients with infrequent and frequent recurrences. Serum IL-4 levels in patients with infrequent and frequent recurrences were 8.5 times and 22.5 times, respectively, higher than reference values. During recurrence, the mean serum IL-4 level in patients with frequent recurrences was 39% higher than in those with infrequent recurrences. No significant difference was observed in serum IL-10 level between remission and recurrence for patients with infrequent recurrences, with a mean value being 24% higher than reference values. The mean serum IL-10 level (for recurrence and remission) in patients with frequent recurrences was 74% higher than for patients with infrequent recurrences (p = 0.01), and two times higher than reference values. Conclusion: HSK in patients with frequent recurrences was characterized by the pattern of regulation of cytokine expression, with decreased expression of TNF-α and increased expression of pro-inflammatory (IL-6) and anti-inflammatory (IL-10 and IL-4) interleukins, which might reflect a mechanism of autoimmune response in such a course of herpetic keratitis. Keywords: herpetic stromal keratitis, cytokines, TNF-α, IL-10, IL-10, IL-4
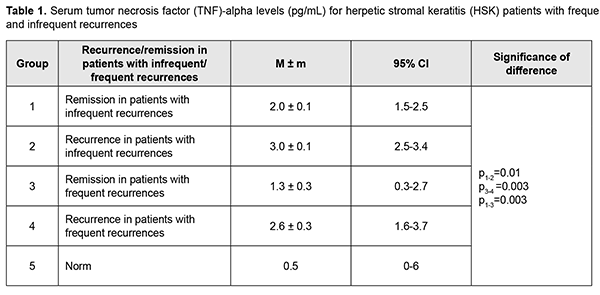
Introduction Herpes simplex virus ocular infection is the major cause of corneal blindness in developed countries [1]. The methods used for the treatment of recurrent herpes infections give only temporary remission, and the results of studies on prevention and prediction of the course of the disease are contradictory, which makes management of these infections not only medically, but also socially important [2]. Herpetic keratitis (HK) is a chronic immunoinflammatory condition that develops in corneal tissue in response to recurrent HSV infection of the cornea [3]. Primary infection results after direct contact of mucosal membrane with herpes simplex virus (HSV)-1 and involves viral replication in the cornea [4]. HSV-1 enters into a host cell through a multistep process as a result of fusion between the viral envelope and target plasma membrane through the interactions of the HSV-1-encoded glycoproteins with their cognate receptors [5]. Shortly after replicating at the initial site of infection, the virus uses retrograde axonal transport to gain access to the sensory neurons in the trigeminal ganglia, where it establishes latent infection. Upon reactivation, newly created particles of virus travel down sensory trigeminal nerve fibers to epithelial surfaces where they replicate locally [6]. Herpes simplex keratitis can be divided into preclinical (week 1 postinfection) and clinical phases (10–20 days post-infection). Corneal infiltration begins in preclinical period, but maximum cloudiness does not occur until the clinical phase [7]. Immunological damage is biphasic and primarily results from CD4+ T lymphocytes and neutrophil migration into the cornea [4, 8]. HSV infection can cause a range of changes to the cornea, from as mild as corneal epitheliopathy to as severe as stromal keratitis, keratouveitis and neurotrophic keratitis [9]. In humans, herpetic stromal keratitis (HSK) is characterized by a mixed infiltrate composed of chronic inflammatory cells, including lymphocytes, neutrophils, and mononuclear phagocytes [10]. Multiple cell types (e.g., natural killer (NK) cells, dendritic cells, macrophages, neutrophils and T-cells) have been shown to contribute to the host innate and adaptive immune responses to virus in a murine model of HK infection [11]. These cells are attracted to the site of HSV lesions and activated by cytokines and chemokines [11]. The damage to corneal tissue is caused largely by the presence of overwhelming numbers of neutrophils and CD4 T cells in inflamed cornea. Numerous pathological processes are triggered, leading to irreversible tissue damage and scarring. Consequently, strategies to promote viral clearance, reduce the numbers of neutrophils, and reduce the numbers of CD4 T cells in inflamed corneas could be highly effective in controlling virus-induced corneal inflammation [3]. Cytokines are small proteins secreted by cells which play an important proinflammatory or anti-inflammatory role in modulating the disease process. Categories of cytokines include interferons, TGF-β, interleukins, and chemokines [12]. The literature is scarce on the effect of cytokines on neutrophil infiltration in HK [13]. The actual tissue damage is the consequence of inflammatory events that derive primarily from neutrophils, the most abundant cell type in lesions at all phases of HSK pathogenesis. The massive cellular infiltration especially neutrophils coupled with the inflammatory mediators secreted by the immune cells are primarily responsible for the swelling and destruction of the cornea [14]. IL-8 and IL-17 are major cytokines that promote cell attraction. IL-17 induces neutrophil chemoattractants, acts as a neutrophil survival factor and also may drive the cells to produce and release tissue damaging molecules such as matrix metalloproteinases and oxyradicals. A reduced infiltration of neutrophils in the cornea was found after IL-17 neutralization in mice [15]. IL-17 plays an important role in the migration of neutrophils in herpes simplex keratitis, but plays no role in viral replication in the cornea [16]. Th17 cells produce IL-17; IFN-γ on the other hand suppresses Th17 cells and IL-17 expression [15]. IL-17 also induces the production of IL-6, a proinflammatory cytokine with an autocrine and paracrine action, which in turn has been found to induce corneal cells to produce MIP-2 and MIP-1α, potent chemoattractants for neutrophils. In the murine model of HK [7], the most prominent cytokines detected were IL-1 alpha and IL-6. Both were elevated by day 2 after infection, reached peak levels at day 10, and then diminished over the next 10 days. Naive corneas cultured in vitro spontaneously produced IL-1 and IL-6, indicating that cells resident in the cornea had the ability to synthesize these proinflammatory cytokines. IL-4 was detected at days 4 to 14 after infection with HSV-1 [7]. Both in vivo and in vitro data indicate that IL-6 produced from virus-infected cells can stimulate non-infected resident corneal cells and other inflammatory cells in a paracrine manner to secrete VEGF, a potent angiogenic factor. Antibody neutralization of IL-6 resulted in a significant decrease in the number of VEGF producing cells in the cornea. The results of the study further demonstrated the close relationship between proinflammatory cytokines and VEGF-induced corneal neovascularization [17]. Inhibition of IL-10, an anti-inflammatory cytokine, causes increased levels of IL-6, consequently leading to increased production of MIP-2 and MIP-1α which then attracts neutrophils and increases the severity of the lesion. MIP-1α helps attract both neutrophils and T cells and is produced by numerous cells including neutrophils, macrophages, and T cells [18]. Lower MIP-1α levels and severity of corneal lesion was found in murine cornea with lower IL-2 и IFN-γ levels [19]. Increased levels of IL-2, IFN-γ and TNF-α were found in mice with induced HSV infection. IL-2 can induce production of IFN-γ and TNF-α, activate neutrophils and prevent their apotosis [20]. Locally produced IL-10, an anti-inflammatory cytokine, acts in an autocrine and paracrine fashion, limits production of proinflammatory mediators, thus limiting corneal inflammation and leading to reduced tissue infiltration with neutrophils [21,22]. Vascular endothelial growth factor (VEGF)-stimulated ocular neovascularization caused by HSV is a characteristic of the severity of corneal lesion. The newly formed vessels play a dual role. First, they supply the inflamed cornea with oxygen and nutrients, and second, they carry pro- and anti-inflammatory components. It has been demonstrated that, in herpetic keratitis, the magnitude of hypoxia correlated with the extent of neutrophils infiltrating the infected corneas, and the depletion of neutrophils reduced the development of hypoxia in infected corneas [23]. Normal cornea has VEGF present, but it binds to the soluble form of the VEGF receptor 1 (sVEGFR-1) preventing VEGF from acting in the cornea to create new blood vessels. Imbalance between concentrations of VEGF and sVEGFR-1 results in excess VEGF leading to angiogenesis [24]. During inflammation, corneal cells (like epithelial cells and fibroblasts and infiltrating cells like macrophages) can produce pro-angiogenic growth factors like vascular endothelial growth factors (VEGF-A, -C and -D) that affect angiogenesis [25]. Increased VEGF-А expression and decreased sVEFGR-1 levels have been shown in HSV infection [17,25]. It has been reported that an imbalance between the levels of VEGF and sVEGFR-1 was induced by over-expression of IL-17, IL-6, and IL-1. These factors caused an increased production of VEGF-А, whereas IL-17 promoted the expression of MMP-2, MMP-8 and MMP-9, the matrix metalloproteinases that degrade sVEGFR-1. Therefore, both pro- and anti-inflammatory cytokines and chemokines play an important role in the pathogenesis of herpetic keratitis. Cytokines such as IL-17, IL-6, IL-1α, and IFN-γ and chemokines such as MIP-2, MCP-1, MIP-1α, and MIP-1β have proinflammatory role in the destruction caused by HSV including neutrophil infiltration and corneal inflammation, and other chemokines and cytokines such as IL-10 and CCL3 can have a protective role [13]. In addition, the effect of cytokines on corneal neovascularization has been studied in corneal angiogenesis models, and was shown to lead to an imbalance between production and reception of VEGF-А. HSV-host cell interaction activates the inflammatory cascade responsible not only for the outcome of virus infection, but also for progressive corneal opacification due to infiltration of inflammatory cells, angiogenesis and dystrophic nerve endings in the cornea. Current antiviral treatment modalities are targeted to inhibition of viral replication in order to reduce disease duration, severity and recurrence, but these treatments have limitations. Blocking some pro-inflammatory cytokines with antibodies was shown to slow the progression of HSK in animals, and this approach is being tried in humans to develop treatments for the disease. The therapeutic approaches targeted at inhibiting entry of HSV into cells, protein synthesis and VEGF pathways showed promise for treatment of recurrent HSV keratitis in animal studies [26]. There have been a few clinical studies of serum and tear cytokine profiles in patients with various forms of ocular herpes [2,27]. Therefore, the mechanism of recurrence of HSK infection has not been clearly understood, since the disease involves complex pathophysiological mechanisms and immune factors (including cytokines) are complexly interrelated in the pathogenesis. The purpose of the study was to assess serum levels of proinflammatory cytokines (TNF-α and IL-6) and anti-inflammatory cytokines (IL-10 and IL-4) in ocular herpes (herpetic stromal keratitis) patients with frequent and infrequent recurrences during remission and recurrence. Material and Methods Thirty-three in-patients (15 patients and 18 patients experiencing less than and more than one recurrence annually, respectively) treated for recurrent HSK at the Department of Corneal Disorders of the Filatov Institute were included in the study. Disease duration varied from 12 months to 32 years. Mean patient age was 41±1.7 years. Patients underwent a general eye examination including visual acuity, biomicroscopy, ophthalmoscopy, refractometry, visual fields, electrical phosphene threshold, and IOP measurement. Laboratory study techniques Thirty-three patients with recurrent HSV stromal keratitis (15 patients and 18 patients experiencing less than and more than one recurrence annually, respectively) underwent serum testing for IL-4, IL-6, IL-10 and TNF-α at the immunology laboratory of the Filatov Institute. Morning fasting blood samples were collected from the cubital vein. Blood was centrifuged after clotting at 3000 rpm for 10 minutes, and serum was stored at -20 оC for analysis. Serum IL-4, IL-6, IL-10 and TNF-α levels were determined using enzyme-linked immunosorbent assay (ELISA) kits from Vektor-Best (Novosibirsk, Russia) according to the manufacturer’s instruction. Photometric measurements were performed on an ELISA plate reader (Stat Fax 2100, Awareness Technologies Inc, Palm City, FL). Reference normal values were taken from the manufacturer’s instructions. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The Kolmogorov-Smirnov test was used to check for the normality of data distribution for all the variables. Median, interquartile range, means and standard deviation (SD) values were calculated. Mann-Whitney, Kruskal-Wallis and Student tests were used for pairwise comparisons. Results It is worthy of note that both in patients with infrequent recurrences of keratitis and in those with frequent recurrences of keratitis, the mean serum TNF-α level was 50% higher during recurrence than during remission (3.0 ± 0.1 pg/mL vs 2.0 ± 0.1 pg/mL, p = 0.01; and 2.6 ± 0.3 pg/mL vs 1.3 ± 0.3 pg/mL, p = 0.003, respectively, Table 1). In addition, during remission, the mean serum TNF-α level in the former patients was 35% higher (p = 0.003) than in the latter patients.
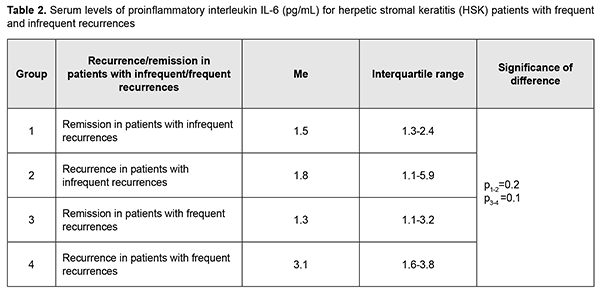
Moreover, the mean serum TNF-α level in patients with infrequent recurrences and in those with frequent recurrences during remission was 2.6 times and 4 times, respectively, higher (Table 1), and during recurrence, 5.6 times higher, compared to normal values. Therefore, in our patients, the level of proinflammatory cytokine TNF-α during remission was significantly higher compared to normal values, which is likely to indicate a subclinical inflammatory process. Serum IL-6 levels were highly variable across patients (range, 0-40 pg/mL), with a percent coefficient of variation of 181%. No significant difference in median serum IL-6 level was seen between groups with frequent and infrequent recurrences, and a median serum IL-6 level [IQR] was 1.3[0-3.5] pg/mL during remission, and increased to 1.3[0-3.5] pg/mL during recurrence (p = 0.09). The mean serum IL-6 level during remission was 2.4 ± 1.0 pg/mL, and increased 2.6 times during recurrence to 6.3 ± 1.8 pg/mL, which was 3 times higher than the norm (2.0 pg/mL). Therefore, serum IL-6 level was substantially increased during recurrence, especially in patients with frequent recurrences, who had the highest median serum IL-6 level (3.1 pg/mL). A high variability of serum IL-6 level among patients of all groups indicates that this cytokine has an intricate mechanism of regulation of expression, with potential involvement of multiple factors.
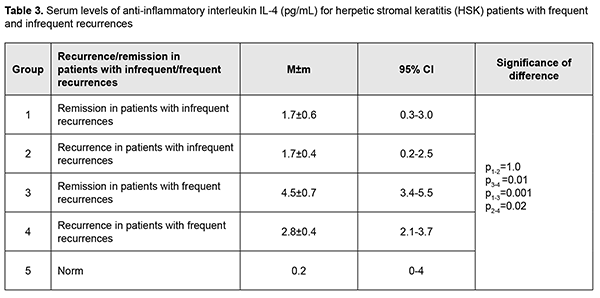
The mean serum IL-4 level in patients with infrequent recurrences during was 1.7 ± 0.4 pg/mL, and was practically similar for recurrence and remission (Table 3).
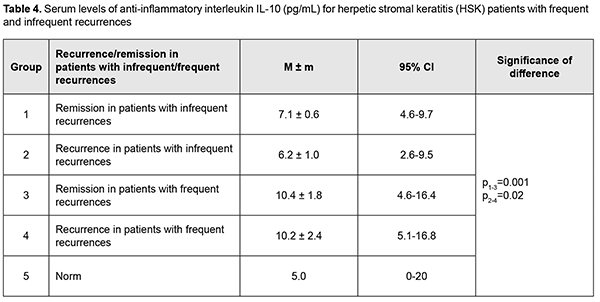
The mean serum IL-4 level in patients with frequent recurrences (4.5 ± 0.7 pg/mL) during remission was 2.6 times higher than in those with infrequent recurrences, showing a 38% decrease to 2.8 ± 0.4 pg/mL during recurrence (p = 0.01). Of note that during recurrence the mean serum IL-4 level in the former patients was 39% higher than in the latter patients (p = 0.02; Table 3). In addition, serum IL-4 levels in HK patients with infrequent and frequent recurrences were 8.5 times and 22.5 times, respectively, higher than reference values (Table 3). No significant difference was observed in serum IL-10 level between remission and recurrence for patients with infrequent recurrences, with a mean value of 6.2 ± 0.8 pg/mL, which was 24% higher than reference values. Moreover, no significant difference was observed in serum IL-10 level between patients with frequent recurrences during remission and patients with infrequent recurrences during recurrence, with a mean value of 10.8 ± 1.8 pg/mL, which was 74% higher than for patients with infrequent recurrences (p = 0.01), and two times higher than reference values (Table 4).
Discussion Recurrent herpetic stromal keratitis is characterized by an inflammatory response that includes neutrophils, macrophages, natural killer cells, and T-cells. The factors that are responsible for this inflammation are proinflammatory cytokines and chemokines. Although many of these factors have been defined for primary disease, relatively few have been investigated during recurrent HSK. Recurrent disease in the cornea is an immunopathologic condition that is initiated by renewed presence of virus in the cornea which re-stimulates the immune response leading to inflammation of the cornea resulting in damage to the cornea [28]. Since an inflammatory host response is a typical pathophysiological response, it contributes to clearance of the infectious agent and infected tissue, but may have destructive effects on the cornea, leading to corneal scarring and neovascularization. Our analysis showed that although no difference was observed in expression direction for studied cytokines, there was a difference in serum levels of these cytokines between patients with HSK having frequent and infrequent recurrences. TNF-α is a potent proinflammatory cytokine secreted most commonly by Th1 cells and also by macrophages. It is a mediator of acute-phase response, chemotaxis and activation of proinflammatory and antigen-presenting cells [29]. We found that the mean serum TNF-α level in patients with infrequent recurrences and in those with frequent recurrences during remission was 2.6 times and 4 times, respectively, higher, and during recurrence, 5.6 times higher, compared to normal values, which is likely to indicate a subclinical inflammatory process. Both in patients with infrequent recurrences and in those with frequent recurrences of keratitis, the mean serum TNF-α level was 50% higher during recurrence than during remission. Our findings are in agreement with a study by Keadle and colleagues [30] who found that TNF-α protein in corneas of mice with recurrent HSK was significantly elevated on days 3 to 10 compared with day 0 levels, and exceeded levels in control corneas on the same days. Hypeproduction of TNF-α in herpetic keratouveitis has been reported [27]. In the current study, we found that, during remission, the mean serum TNF-α level in patients with frequent recurrences was 35% higher (p = 0.003) than in those with infrequent recurrences, which confirmed down-regulation of TNF-α in frequent recurrences and a possibly insufficient anti-inflammatory response, because TNF-α plays an antiviral role in a primary and recurrent acute infection [31]. IL-6 is an important participant in the cytokine cascade triggered by HSV-1 corneal infection, and promotes corneal inflammation by inducing resident corneal cells to make MIP-2 and MIP-1α, which in turn recruit neutrophils to the virus infection site [32]. Results of a study by West and colleagues [28] clearly indicated that unlike primary disease, IL-6 plays no role in recurrent HSK. In the current study, serum IL-6 levels were highly variable across patients, with a percent coefficient of variation as high as 181%. We found that the mean serum IL-6 level during remission was comparable to, and during recurrence was 3 times higher than the norm, with no significant difference between patients with infrequent and frequent recurrences. We conducted an analysis of expression of IL-4 and IL-10, major anti-inflammatory cytokines. IL-4 is known to suppress the expression of inflammatory cytokines and prevent HSV1-induced corneal scarring. What is more important is that IL-4 can reduce viral replication in the cornea [33]. In the current study, serum IL-4 levels in HK patients with infrequent and frequent recurrences were 8.5 times and 22.5 times, respectively, higher than reference values. In addition, during recurrence, the mean serum IL-4 level in patients with frequent recurrences was 39% higher than in those with infrequent recurrences. IL-10 can be expressed by corneal epithelial cells and fibroblasts; it is an endogenous immunosuppressive cytokine and plays a protective role in limiting the HSV1-induced inflammatory response. One anti-inflammatory mechanism of IL-10 is to suppress the proliferation of CD4+ and CD8+ T cells and the production of inflammatory cytokines and chemokines such as IL-2, IL-6, and MIP-1α [34]. IL-10 contributes to immune tolerance, resolution of inflammation and apoptosis in systemic and ocular disorders [35]. In the current study, no significant difference was observed in serum IL-10 level between remission and recurrence for patients with infrequent recurrences, with a mean value being 24% higher than reference values. This was in contrast with the findings in patients with frequent recurrences. Although no significant difference was observed in serum IL-10 level between remission and recurrence for these patients, their mean serum IL-10 level value was 74% higher than for patients with infrequent recurrences and two times higher than reference values. IL-10 is an immunoregulatory cytokine in autoimmune diseases and an immunosuppressive cytokine inhibiting production of chemokines [36]. Several lines of evidence have been reported that IL-10 family cytokines are involved in autoimmune diseases. Among the IL-10 family cytokines analyzed, IL-19 demonstrated the highest expression in endogenous uveitis, particularly in HLA-B27-associated uveitis. Deficient levels of IL-10 have been reported in individuals with keratitis [27]. We noted an increased expression of IL-10 in recurrent HSK, particularly in patients with frequent recurrences, which seems to suggest that an autoimmune mechanism is involved in the pathogenesis of this form of HK. Of note is that during remission in HSK patients with frequent and infrequent recurrences, the levels of pro-inflammatory (TNF-α) and anti-inflammatory (IL-10 and IL-4) cytokines were higher than normal values, which supposes a subclinical inflammatory process. In this study, we found that HSK in patients with frequent recurrences was characterized by special pathophysiological mechanisms for regulation of inflammatory response, with decreased expression of TNF-α and increased expression of pro-inflammatory (IL-10) and anti-inflammatory (IL-10 and IL-4) interleukins, which might be a mechanism of autoimmune response in such a course of herpetic keratitis.
References 1.Darougar S, Wishart MS, Viswalingam ND. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol. 1985 Jan; 69(1):2-6. 2.Cherevko NA. [Immunological mechanisms of reactivation of herpetic infection its relationship with allergopathology]. Dr Sc (Med) Dissertation. Tomsk: Siberian State Medical University; 2012. Russian. 3.Gaddipati S., Estrada K., Rao P., Jerome A. D., Suvas S. IL-2/anti-IL-2 antibody complex treatment inhibits the development but not the progression of herpetic Stromal Keratitis. J Immunol. 2015;194(1):273–82. 4.Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J Immunol. 1997 Feb 1; 158(3):1275-83. 5.Karasneh GA, Shukla D Herpes simplex virus infects most cell types in vitro: clues to its success. Virol J. 2011 Oct 26; 8():481 6.Miller CS, Danaher RJ, Jacob RJ. Мolecular aspects of herpes simplex virus I latency, reactivation, and recurrence. Crit Rev Oral Biol Med. 1998; 9(4):541-62. 7.Staats HF, Lausch RN Cytokine expression in vivo during murine herpetic stromal keratitis. Effect of protective antibody therapy. J Immunol. 1993 Jul 1; 151(1):277-83. 8.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–91. 9.Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Hossain P, Anderson D Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol. 2016 Dec; 44(9):824-37. 10.Liesegang TJ. Ocular herpes simplex infection: pathogenesis and current therapy. Mayo Clin Proc. 1988;63:1092–105). 11.Frank GM, Buela KA, Maker DM, Harvey SA, Hendricks RL. Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea. J Immunol. 2012;188:1350–1359 12.Zhang J, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. Spring 2007;45(2):27-37. doi: 10.1097/AIA.0b013e318034194e. 13.Azher TN, Yin XT, Stuart PM. Understanding the Role of Chemokines and Cytokines in Experimental Models of Herpes Simplex Keratitis. J Immunol Res. 2017;2017:7261980. 14.Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res. 1998 Dec;67(6):619-24. 15.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011 Aug 15;187(4):1919-30. 16.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008 Feb;83(2):401-8. 17.Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exper Eye Res. 2006 Jan;82(1):46-54. 18.Yan XT, Zhuang M, Oakes JE, Lausch RN. Autocrine action of IL-10 suppresses proinflammatory mediators and inflammation in the HSV-1-infected cornea. J Leukoc Biol. 2001 Jan;69(1):149-57. 19.Tumpey TM, Cheng H, Cook DN, Smithies O, Oakes JE, Lausch RN. Absence of macrophage inflammatory protein-1α prevents the development of blinding herpes stromal keratitis. J Virol. 1998;72(5):3705–3710. 20.Tang Q, Hendricks RL. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J Exp Med. 1996;184(4):1435–47. 21.Jan XT, Zhuang M, Oakes JE, Lausch RN. Autocrine action of IL-10 suppresses proinflammatory mediators and inflammation in the HSV-1-infected cornea. J Leukoc Biol. 2001 Jan;69(1):149-57. 22.Tumpey TM, Cheng H, Yan XT, et al. Chemokine synthesis in the HSV-1 infected cornea and its suppression by interleukin-10. J Leukoc Biol. 1998;63:486-92. 23.Rao P, Suvas S. Development of Inflammatory Hypoxia and Prevalence of Glycolytic Metabolism in Progressing Herpes Stromal Keratitis Lesions. J Immunol. 2019;202(2):514-526. 24.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, Cursiefen C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013 May; 34():89-124. 25.Wuest T. R., Carr D. J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. The Journal of Experimental Medicine. 2010;207(1):101–115. 26.Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019 Jan;17(1):40-49. 27.Derbasova NN. [Features of immune impairments in ocular herpetic infection and clinical and immunological efficacy of various therapeutic regimens]. Abstract of Cand Sc (Med) Thesis. Vladivostok: Vladivostok State Medical University; 2007. Russian. 28.West DM, Del Rosso CR, Yin XT, Stuart PM. CXCL1 but not IL-6 is required for recurrent herpetic stromal keratitis. J Immunol. 2014;192(4):1762-67. 29.Sekine-Okano M, Lucas R, Rungger D, et al. Expression and release of tumor necrosis factor-alpha by explants of mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1302–10. 30.Keadle TL, Usui N, Laycock KA, Miller JK, Pepose JS, Stuart PM. IL-1 and TNF-alpha are important factors in the pathogenesis of murine recurrent herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2000;41 (1):96-102. 31.Minagawa H, Hashimoto K, Yanagi Y. Absence of tumour necrosis factor facilitates primary and recurrent herpes simplex virus-1 infections. J Gen Virol. 2004 Feb;85(Pt 2):343-7. 32.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002 Mar;43(3):737-43. 33.Ghiasi H, Cai S, Slanina SM, Perng GC, Nesburn AB, Wechsler SL. The role of interleukin (IL)-2 and IL-4 in herpes simplex virus type 1 ocular replication and eye disease. J Infect Dis. 1999 May;179(5):1086-93. 34.Sarangi PP, Sehrawat S, Suvas S, Rouse BT. IL-10 and natural regulatory T cells: two independent anti-inflammatory mechanisms in herpes simplex virus-induced ocular immunopathology. J Immunol. 2008 May 1; 180(9):6297-306. 35.Atan D, Fraser-Bell S, Plskova J, Kuffova L, Hogan A, Tufail A, et al. Cytokine polymorphism in noninfectious uveitis. Invest Ophthalmol Vis Sci. 2010 Aug; 51(8):4133-42. 36.Ghasemi H, Ghazanfari T, Yaraee R, et al. Roles of IL-10 in ocular inflammations: a review. Ocul Immunol Inflamm. 2012 Dec;20(6):406-18. 37.Abu El-Asrar AM, Berghmans N, Al-Obeidan SA, et al. Expression of interleukin (IL)-10 family cytokines in aqueous humour of patients with specific endogenous uveitic entities: elevated levels of IL-19 in human leucocyte antigen-B27-associated uveitis. Acta Ophthalmol. 2019;97(5):e780-e784.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|