J.ophthalmol.(Ukraine).2021;6:77-83.
|
http://doi.org/10.31288/oftalmolzh202167783 Report on the clinical study of glautan and original travoptost in patients with primary open angle glaucoma Soboleva IA, Dr Sc (Med), Prof. Kharkiv Medical Academy of Postgraduate Education TO CITE THIS ARTICLE: Soboleva IA. Report on the clinical study of glautan and original travoptost in patients with primary open angle glaucoma. J.ophthalmol.(Ukraine). 2021;6:77-83. http://doi.org/10.31288/oftalmolzh202167783
Open angle glaucoma is characterized by specific lesions of the optic disc and is accompanied by visual field loss in the presence of elevated intraocular pressure (IOP). The management of glaucoma is challenging, the disease has become a matter of rising social and economic concern and a major cause of the deterioration in quality of life in patients [1, 2]. It is important that glaucoma is highly prevalent among economically-active working-age individuals and is the most common cause of certified visual disability [3, 4]. Glaucoma incidence increases with age. Primary open angle glaucoma (POAG) is more common in individuals older than 40 years of age. Hydromechanical, blood circulation, degenerative and destructive, immune and other abnormalities are involved in the mechanisms of the disease. POAG is a multifactorial optic neuropathy characterized by progressive loss of retinal ganglion cells and their axons leading to apoptosis [1, 5, 6]. Currently, there are three major theories (mechanical, vascular and metabolic theories) of glaucomatous optic neuropathy (GON) [1, 4]. The mechanical theory suggests that GON may result from increased IOP leading to regions of high shear stress and strain in the lamina cribrosa and damage to retinal ganglion cell axons. The vascular theory of glaucoma considers GON as a consequence of impaired blood flow to the optic disc. The major cause of insufficient blood supply to the optic disc is impaired vascular regulation that results in reduced retinal perfusion and abnormal local self-regulation, leading to increased optic nerve sensitivity and IOP fluctuations. When a combination of mechanical and vascular factors occurs, it may activate metabolic processes. Research is ongoing on the role of metabolic abnormalities and the impact of free radicals, lipoperoxides, vasoactive peptides and nitric oxide on the components of the ocular drainage system, their damaging effect and the associated elevated IOP. Nerve tissue ischemia promotes the formation of excessive free radicals and activation of lipid peroxidation. This triggers the cascade of pathological biochemical processes which exert a cytotoxic effect on the retina and optic nerve, leading to glaucomatous optic neuropathy in the form of apoptosis of retinal ganglion cells and damage to optic nerve fibers [6, 7]. Nevertheless, elevated IOP is the major risk factor of the development of the disease, causes optic nerve damage, and is the major treatable factor. Glaucoma is characterized by the loss of retinal ganglion cells and their axons, which form the retinal nerve fiber layer (RNFL) [1, 8]. The loss of retinal ganglion cells cannot be seen by a routine direct ophthalmoscopy. In addition, during clinical examination, it is difficult to detect defects of nerve fiber plexuses. Moreover, red-free fundus photography gives only a rough estimate of the state of these plexuses, and is rarely used in clinical practice. Glaucoma diagnosis traditionally involves the assessment of optic disc excavation and relevant visual field defects [9, 10]. Loss of retinal ganglion cells take place long before the initial signs of glaucomatous changes in visual fields appear, and structural abnormalities may occur as much as five years before the onset of clinical abnormalities [2, 3]. Initial clinical changes in visual fields appear only when there is as much as a 30% loss of optic nerve fibers [4, 10]. Therefore, the development of novel objective methods for quantitative assessment the glaucomatous changes induced by the loss of retinal ganglion cells would enable earlier detection of glaucoma and objective assessment of longitudinal changes in the disease state. A significant portion of the population of retinal ganglion cells resides in the macular region, where the cell bodies form as much as seven layers. Approximately 50% of retinal ganglion cells reside in the perifovea region [5, 7]. Zeimer and colleagues [7] were the first to describe retinal thinning at the macula in glaucoma. Later studies, however, demonstrated that optical coherence tomography (OCT)-derived total retinal thickness at the macula is less helpful in the diagnosis of glaucoma than the parameters of the peripapillar RNFL [6, 11]. Glaucoma presumably affects the three internal retinal layers (the RNFL, retinal ganglion cell layer and internal plexiform layer) that contain axons, cell bodies and dendrites, respectively, of retinal ganglion cells. Tan and colleagues [12] coined the term “ganglion cell complex” (GCC) to name the aggregate of these layers. The accurateness of OCT assessment for glaucoma will improve if macular thickness measurements are performed only in the inner retinal layers [11]. The advent of the spectral-domain OCT (SD-OCT) has allowed for the estimation of the thickness of particular retinal layers. SD-OCT has faster acquisition time and higher resolution than time-domain (TD)-OCT. Fast acquisition enables highly detailed maps of the retina (i.e., the maps including vast retinal areas with numerous examination points) to be acquired. Highly detailed maps facilitate the identification of retinal layers and their separation from each other (particularly, separation of the ganglion cell complex) [2, 3]. Therefore, glaucoma treatment should be targeted at neuroprotection as well as IOP reduction (by medicamentous, laser and surgical methods). Ocular hypotensive medications work by reduction of aqueous humor production or enhancement of aqueous humor outflow. Pathogenetically, the glaucoma therapy enhancing aqueous humor outflow is believed to be more beneficial than that reducing aqueous humor production, because aqueous humor hypersecretion is very uncommon, whereas the pathogenesis of most forms of glaucoma involves impaired aqueous humor outflow [5, 7]. The mechanism of action of a certain number of ocular hypotensive medications involves activation of aqueous outflow. Prostaglandin analogues are the most effective hypotensive medications and are typically recommended as a first-line therapy for glaucoma [1, 3, 7]. An improvement in our understanding of uveoscleral outflow of aqueous humor is associated with the development of this type of hypotensive medications [6]. The major effect of prostaglandin analogues is mediated via the FP receptors, leading to enhanced ciliary muscle permeability and improved aqueous outflow through the intermuscular space [6, 7]. Others have not found a significant impact of prostaglandin analogues on aqueous humor production and episcleral venous pressure [5, 6]. Prostaglandins are thought to stimulate the synthesis of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPS). The MMPs to TIMPs ratio is a factor that determines tissue resistance to fluid flux, since it regulates the permeability of intercellular substance in connective tissue. Changes in the extracellular matrix of the ciliary muscle (like an increase in the spacing between filaments and loss of type I and type III collagen) can be noted in the presence of treatment with prostaglandins [3, 7]. Such morphology can be seen in the ciliary body of young adults, with their extracellular matrix being looser and spacing between filaments greater than those in the ciliary body of senior individuals. Consequently, the first-line therapy with prostaglandin analogues is pathogenetic for glaucoma. However, the system of MMPs and their TIMPs is widely represented not only in the ciliary muscle, but also in the trabecular meshwork [3, 5]. Therefore, prostaglandins exert an effect on the anterior segment structures (like the trabecular meshwork and the ciliary muscle) involved in aqueous humor outflow. This produced an opinion that not only activation of uveoscleral outflow of aqueous humor may take place, but also activation of trabecular outflow of aqueous humor might take place [7]. Indeed, the trabecular meshwork is a complex 3-D structure enabling aqueous humor passage from the anterior chamber over the two routes [3]. The first route is through oval pores in trabecular plates and juxtacanalicular tissue to the lumen of Schlemm’s canal. Here the juxtacanalicular tissue represents the main barrier, with its permeability determined by the balance between synthesis and scavenging of glycosaminoglycans. The second route provides fluid passage over pore-like spaces amidst trabecular layers which are filled by amorphous matrix and continuously traverse the spaces between ciliary muscle fibers. Consequently, it is possible that the impact of prostaglandins on the receptors located in the trabecular meshwork causes an effect on aqueous outflow over the two routes. Therefore, it appears that prostaglandine analogs reduce IOP not exclusively by increasing uveoscleral outflow facility, but predominantly by increasing uveoscleral outflow and to a lesser extent by trabecular outflow facility [3, 6, 7]. Travoprost is an analogue of prostaglandin F2a. It is a selective FP prostaglandin full receptor agonist with high affinity at the FP receptor. It reduces IOP by increasing uveoscleral outflow and trabecular outflow facility. Travoprost is an ester prodrug. It is absorbed through the cornea where the isopropyl ester is hydrolysed to the active free acid. Increased MMP synthesis with a prostaglandin-induced reduction in the amount of intercellular matrix was demonstrated in human trabecular cell culture [10]. This may result in hydrolysis of collagen and fibronectin in these structures. The purpose of the study was to compare the efficacy of travoprost 0.004% (GLAUTAN; Farmak JSC, Kyiv, Ukraine) with that of original travoprost 0.004% (Travatan®; Alcon Laboratories, Inc., Ft Worth, TX, USA) in patients with glaucoma. Travoprost is a synthetic analogue of prostaglandin F2a. In addition, it is a highly selective agonist of the prostaglandin FP receptor and reduces IOP by increasing uveoscleral outflow and trabecular outflow facility. Material and Methods The study design envisages the creation of two randomized groups of patients each, Group 1 (or the main group) and Group 2 (or the control group). Group 1 included 25 patients with POAG receiving topical eye drops of GLAUTAN, and Group 2 included 25 patients with POAG receiving topical original travoptost (hereinafter, the reference medication). Fifty patients (84 eyes) with primary open angle glaucoma aged from 40 to 75 years (mean age: 63.2 ± 2.2) were under our care. Of the 84 eyes, 34 (40.4%) were diagnosed with mild glaucoma, 28 (33.4%), with moderate glaucoma, and 22 (26.2%), with advanced glaucoma. Thirty-eight eyes (45.2%) had a moderately increased IOP (32 mmHg or lower), and 46 eyes (54.8%), elevated IOP (32 mmHg to 42 mmHg). Patients were divided into two groups. Group 1 included 25 patients (44 eyes) with POAG stage 1 or stage 2 who received one drop a day of topical Glautan. Group 2 included 25 patients (40 eyes) with POAG stage 1 or stage 2 who received one drop a day of reference medication. Patients underwent visual acuity assessment, biomicroscopy, study of the hydrodynamics of the eye, tonometry, tonography, evaluation of ocular blood supply, and gonioscopy. The Goldmann three-mirror lens was used to perform gonioscopy. This lens has one mirror dedicated to viewing the angle of the anterior chamber, and two others for examination of the peripheral retina and central retina. In addition, the lens provides a 3D image of the optic nerve and fundus, which facilitates making a correct diagnosis of glaucoma and identifying the stage of the disease. Static perimetry was performed using Humphrey Field Analyzer II (HFA-II, 30-2 SITA standard, Carl Zeiss AG, Jena, Germany). Threshold values of 176 points of the central 30 degrees were determined with the perimeter's standard white round stimulus (Goldmann Size III), stimulus duration of 100 msec, and moderately intense bowl illumination (31.5 asb). Mean deviation (MD) and pattern standard deviation (PSD) values as well as p values for them were determined. Reliability of visual field results was established based on the proportions of false-positive response errors, false-negative response errors, and fixation losses. The RTVue-100 ОСТ (Optovue, Inc., Fremont, CA) system was used to perform OCT of the optic disc (ONH and 3D Disc protocols) and the macula (GCC protocol). In brief, the latter protocol is as follows. The ganglion cell complex (GCC) scan, which covers a 7 x 7 mm area of the macula, is centered 1 mm temporal to the fovea (i.e., the area of maximum concentration of ganglion cells). A special set of optical scans produces 14,994 measurements within 0.58 sec at 16 lines (15 parallel vertical lines and a horizontal line) in the above area. The GCC protocol explores parameters within a circle with a 6 mm diameter which conforms to a 16^20° field of vision. In addition, average superior GCC thickness, average inferior GCC thickness and mean average GCC thickness for the superior and inferior segments are calculated. In the current study, scans with artifacts due to eye movement (nystagmus) or those with a signal strength index ≤45 were excluded to not affect the accuracy of the determination of the margins of the retinal layers. The following optic nerve head (ONH) parameters were examined: Disk Area, Cup/Disk (C/D) Vertical Ratio, C/D Horizontal Ratio, C/D Area Ratio, Rim Area, Cup Area, and RNFL thickness. In addition, the GCC protocol was used to obtain Average GCC, Focal Loss Volume (FLV), and Global Loss Volume (GLV) values. Statistical analyses were conducted using Statistica 8.0 (StatSoft, Tulsa, OK, USA) software. Microsoft Excel was used to build correlation curves and receiver operating characteristic (ROC) curves, and calculate the area under curve (AUC).
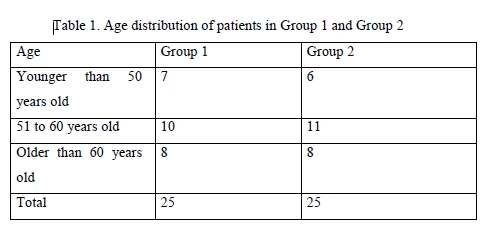
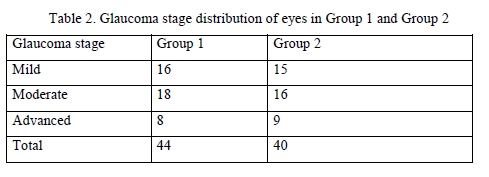
Results There was no significant difference among the two groups with regard to gender and age (Table 1) and stage of glaucoma (Table 2) of the patients. Therefore, the two groups were similar with regard to gender, age, stage of glaucoma, comorbidity and hypotensive therapy protocol.
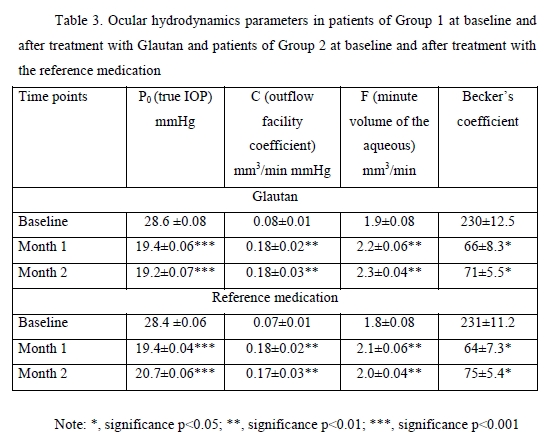
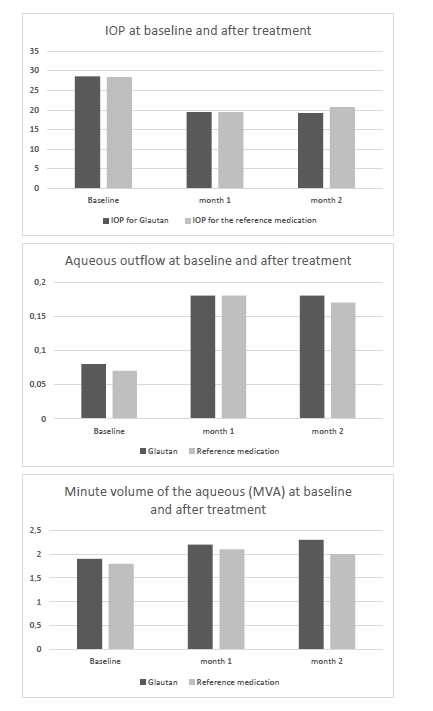
Topical glautan produced an apparent ocular hypotensive effect in most (88%) patients with glaucoma. The IOP reduction, however, was gradual, and a minimum reduction of 0.7-1.0 mmHg was obtained in two hours, with no significant difference with baseline (p > 0.05). A significant IOP reduction by 21% from baseline (p < 0.05) was observed in 8-10 hours, and a maximum IOP reduction of 30% from baseline (p < 0.05), in 22 hours. In 4 patients (5 eyes; 11.3%) of Group 1 and 3 patients (4 eyes; 11.3%) of Group 2, mostly with advanced glaucoma, IOP reduction was obtained 10-14 days after hypotensive treatment. In Group 1, two eyes (4.5%) received non-penetrating deep sclerectomy, and one eye (2%), laser trabeculoplasty for decompensated glaucoma within two months. The treatment with reference medication led to a significant IOP reduction in 87% of patients. The IOP reduction was gradual, and a minimum reduction of 0.6-0.9 mmHg obtained in two hours, with no significant difference with baseline (p > 0.05). A significant IOP reduction by 20% from baseline (p < 0.05) was observed in 8-11 hours, and a maximum IOP reduction of 29% from baseline (p < 0.01), in 24 hours. In Group 2, one eye (2.5%) received non-penetrating deep sclerectomy, and one eye (2.5%), laser trabeculoplasty, due to insufficient hypotensive effect of the reference medication. Therefore, in patients with POAG, topical treatment with GLAUTAN caused a statistically significant reduction in IOP from baseline, which was similar to that in patients treated with the reference medication at particular time points after treatment. The mean reduction in IOP was 7.4-9.0 mmHg for GLAUTAN versus 7.2-8.9 mmHg for the reference medication. The percentage of eyes that achieved an IOP < 18 mmHg was 35-62% for GLAUTAN versus 34–61% for the reference medication. Tonography allows determining the state of the aqueous outflow, making a correct diagnosis, performing treatment monitoring and assessing treatment efficacy. In addition, the method allowed us to confirm the mechanism of hypotensive action, with an increase in the outflow facility coefficient (OFC), both for GLAUTAN and for the reference medication. The OFC value ranged from 0.02 to 0.1 mm3/min mmHg at baseline, and improved to 0.26 at two months after treatment (p < 0.01). Table 3 presents the characteristics of ocular hydrodynamics. Both treatment options (GLAUTAN and the reference medication) resulted in a statistically significant IOP reduction, and, importantly, this reduction was maintained during a follow up as long as 4 months. It is very important that the reduction in IOP was associated not with the inhibition of aqueous production, but with a more than two-fold significant increase (from 0.08 ± 0.01 to 0.18 ± 0.03, p < 0.01) in aqueous outflow.
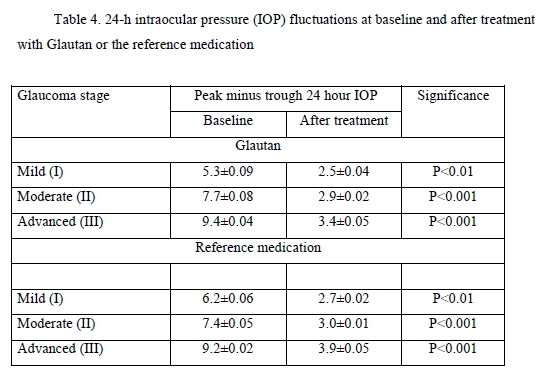
24-h IOP fluctuations are of considerable importance in the treatment of glaucoma. Patients of the study underwent a 24-h IOP monitoring. The results of the monitoring are presented in Table 4.
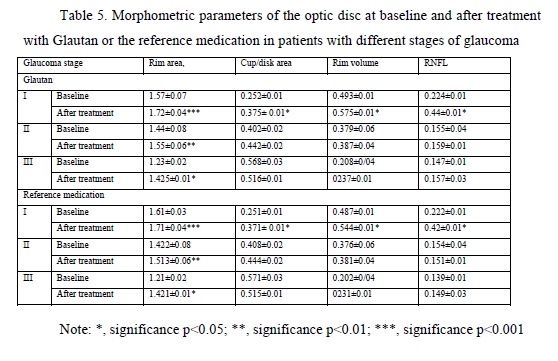
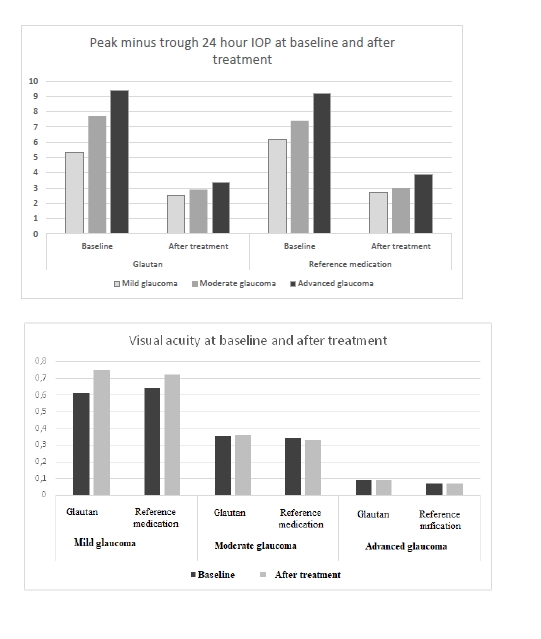
At baseline, the greatest 24-hour IOP fluctuation (defined as peak minus trough 24 hour IOP) was seen in patients with moderate glaucoma and advanced glaucoma. After treatment, in both groups of patients, there was a 2.2-fold decrease in 24-hour IOP fluctuation in mild glaucoma, and a 3-fold decrease in 24-hour IOP fluctuation in moderate glaucoma and advanced glaucoma. Therefore, the impact of GLAUTAN on 24-hour IOP fluctuation in patients with various stages of glaucoma was statistically significant and similar to that found in patients with various stages of glaucoma treated with the reference medication. Optical coherence tomography has become a valuable tool for diagnosis and follow-up of glaucoma. OCT scans enable non-invasive measurements of structural changes in glaucoma. OCT enables quantitative recording and analysis of the optic disc and peripapillary retina at the macula, the locations most affected by glaucoma. We examined changes in amplitudes of morphometric parameters of the optic disc from baseline after treatment with GLAUTAN versus the reference medication. The results of this examination are presented in Table 5.
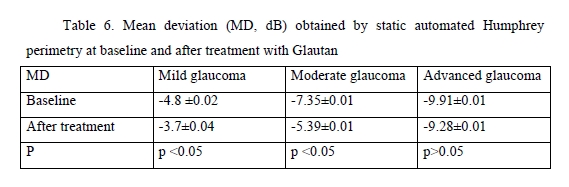
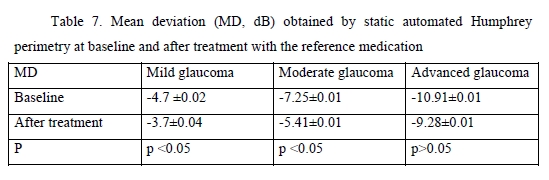
We found significant changes in amplitudes of optic disc parameters, RNFL thickness, neuroretinal rim area and volume, and cup-to-disc ratio, after hypotensive treatment compared to baseline. The best and statistically significant post-treatment improvement was observed in the neuroretinal rim area in patients with different stages of glaucoma, with no significant difference between patients treated with GLAUTAN and those treated with the reference medication. The values of the three indices representing the state of the GCC were also examined (Table 5). There was no significant difference between groups with regard to any of the three parameters (significance by the Kruskal–Wallis test was р > 0.05). There was no significant difference in GCC Average Thickness or Global Loss Volume (GLV) values (significance by the Mann–Whitney test with Bonferroni was р > 0.05) after treatment compared to baseline. Post-treatment changes in optic disc parameters, RNFL thickness, neuroretinal rim area and volume were noted in all patients. In the current study, the best and statistically significant post-treatment improvement was observed for the Rim area in patients with mild glaucoma and moderate glaucoma. All patients underwent static automated Humphrey perimetry at baseline and after treatment. Static perimetry requires presenting a stimulus of varying intensity at a fixed visual field location to determine the threshold sensitivity at that locus. The method allows not only identifying defects, but also determining retinal light sensitivity at specified locations in the visual field. Apostilb (abs) is a unit of luminance of background illumination or stimulus presentation. Measurements expressed in apostilbs are usually converted to decibel (dB) units to express threshold light sensitivity of the retina. Unlike apostilbs, decibels are logarithmic units (reciprocal of log intensity in apostilbs). The results of static automated Humphrey perimetry are presented in Tables 6 and 7.
The threshold light sensitivity of the retina was more than 2.5-fold lower than normal in all patients, with a normal range of 1.5–2.0 dB. After treatment with GLAUTAN, threshold light sensitivity of the retina improved by 18.8% (from 4.8 ± 0.02 dB to 3.7 ± 0.04 dB) in patients with stage 1 of the disease, and improved significantly by 19.0% from a 4-fold lower than normal value (7.35 ± 0.01 dB) to 5.39 ± 0.01 dB in patients with stage 2 of the disease. There was no significant difference between baseline and post-treatment threshold light sensitivity of the retina in patients with advanced glaucoma.
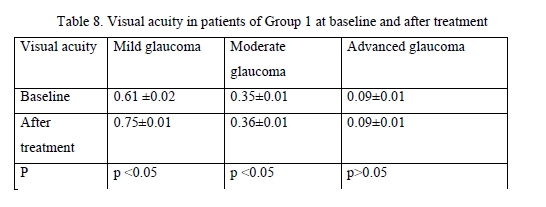
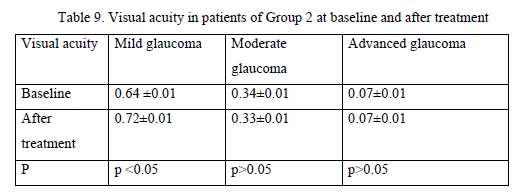
Among patients treated with GLAUTAN, visual acuity increased only in 10 eyes (62.5%) with mild glaucoma, with a 3.1% increase from 0.61 ± 0.02 to 0.72 ± 0.06, but no statistically significant increase in visual acuity was observed in patients with moderate glaucoma or advanced glaucoma. In addition, no deterioration in visual acuity in any patient was observed over the follow-up period. Visual acuities in patients of Group 2 before and after treatment were close to those in patients of Group 1. The results of visual acuity assessment are presented in Table 9.
Therefore, several conclusions can be made based on the results of this study. First, topical GLAUTAN produced an apparent ocular hypotensive effect in patients with POAG, with a 30% significant decrease in IOP (p< 0.05). Second, the reduction in IOP was associated not with the inhibition of aqueous production, but with a more than two-fold significant increase (from 0.08 ± 0.01 to 0.18 ± 0.03, p < 0.01) in aqueous outflow. Third, topical GLAUTAN produced a decrease in 24-hour IOP fluctuation in patients with POAG. After treatment, there was a 2.6-fold decrease in 24-hour IOP fluctuation in mild glaucoma, and a 2.8-fold decrease in 24-hour IOP fluctuation in moderate glaucoma and advanced glaucoma. The medication alleviated 24-hour fluctuations in morphometric parameters of the optic disc and IOP in 78% of patients with mild glaucoma. Fourth, GLAUTAN demonstrated an impact on the morphometric parameters of the optic disc, with a significant increase in RNFL thickness and neuroretinal rim area in patients with stage 1 and stage 2 of glaucoma, which may indicate a neuroprotective effect of the medication. In addition, the amplitude of fluctuations of the morphometric structure of the optic disc tended to decrease in patients with POAG. Finally, after treatment with GLAUTAN, threshold light sensitivity of the retina improved by 18.8% (from 4.8 ± 0.02 dB to 3.7 ± 0.04 dB) in patients with stage 1 of the disease, and improved significantly by 19.0% from a 4-fold lower than normal value (7.35 ± 0.01 dB) to 5.39 ± 0.01 dB in patients with stage 2 of the disease. Overall, GLAUTAN (Farmak JSC, Kyiv, Ukraine), a medication containing travoprost, was found to be similar to the original travoptost medication with regard to its action and effective IOP reduction in eyes with POAG.
References 1. Sommer A, Quigley HA, Robin AL, et al. Evaluation of nerve fiber layer assessment. Arch Ophthalmol. 1984 Dec;102(12):1766-71. doi: 10.1001/archopht.1984.01040031430017. 2. Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991 Jan;109(1):77-83. doi: 10.1001/archopht.1991.01080010079037. 3. Airaksinen PJ, Drance SM, Douglas GR, et al. Diffuse and localized nerve fiber loss in glaucoma. Am J Ophthalmol. 1984 Nov;98(5):566-71. doi: 10.1016/0002-9394(84)90242-3. 4. Kurysheva NI. [Glaucomatous optic neuropathy]. Moscow: Medpressinform; 2006. Russian. 5. Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990 Oct 1;300(1):5-25. doi: 10.1002/cne.903000103. 6. Munemasa Y, Kitaoka Y. Molecular mechanisms of retinal ganglion cell degeneration in glaucoma and future prospects for cell body and axonal protection. Front Cell Neurosci. 2013 Jan 9;6:60. doi: 10.3389/fncel.2012.00060. 7. Zeimer R, Shahidi M, Mori M, et al. A new method for rapid mapping of the retinal thickness at the posterior pole. Invest Ophthalmol Vis Sci. 1996 Sep;37(10):1994-2001. 8. Duker JC, Waheed MK, Goldman DR. Handbook of Retinal OCT. London: Elsevier. 2014. 9. Nesterov AP. [Primary open angle glaucoma: pathogenesis and principles of management]. Rossiiskii meditsinskii zhurnal. Klinicheskaia Oftalmologiia. 2000; 1:4-5. Russian. 10. Zhaboiedov GD, Petrenko OV, Parkhomenko EG. [Comparing tear nitric oxide levels in healthy individuals and patients with glaucoma]. In: [Proceedings of Fyodorov Memorial Lectures-2006]. p. 201-3. Russian. 11. Bagga H, Greenfield DS, Knighton RW. Macular symmetry testing for glaucoma detection. J Glaucoma. 2005 Oct;14(5):358-63. doi: 10.1097/01.ijg.0000176930.21853.04. 12. Tan O, Chopra V, Lu AT, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 2009; 116: 2305–14. doi:10.1016/j.ophtha.2009.05.025.
|