J.ophthalmol.(Ukraine).2022;1:51-57.
|
http://doi.org/10.31288/oftalmolzh202215157 Received: 04 October 2021; Published on-line: 15 March 2022 Assessing the efficacy of various treatment regimens for patients with endocrine ophthalmopathy associated with Graves’ disease Yu. V. Buldygina 1, G. M. Terekhova 1, L. S. Strafun1, I. I. Savosko 1, Z. G. Lysova 1, S. L. Shlyakhtych 2
1 SI "V.P. Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine"; Kyiv (Ukraine) 2 Kyiv City Center of Endocrinology, Clinical Hospital No.3 of the City of Kyiv; Kyiv, (Ukraine) E-mail: yuliya.buldygina@icloud.com TO CITE THIS ARTICLE: Buldygina Yu. V., Terekhova G. M., Strafun L. S., Savosko I. I., Lysova Z. G., Shlyakhtych S. L. Assessing the efficacy of various treatment regimens for patients with endocrine ophthalmopathy associated with Graves’ disease. J.ophthalmol.(Ukraine).2022;1:51-7. http://doi.org/10.31288/oftalmolzh202215157 Purpose: To assess the efficacy of various treatment regimens for patients with EO associated with Graves’ disease based on the retrospective analysis of clinical data, thyroid-stimulating hormone (TSH) receptor autoantibodies (TSHR-Ab) titers and orbital ultrasound imaging findings. Material and Methods: We retrospectively reviewed the medical records (including clinical and laboratory data and findings of ultrasound imaging of retrobulbar adipose tissue) of 155 patients with EO associated with Graves’ disease and either euthyroidism (in the presence of antithyroid therapy) or postoperative compensated hypothyroidism that underwent treatment at Komisarenko Institute for Endocrinology and Metabolism between 2009 and 2019. The duration of EO ranged from 8 months to 36 months. Patients with EO associated with Graves’ disease were medically treated in the presence of stable euthyroidism. Patients were divided into 4 groups based on the glucocorticoid treatment scheme. Group 1 of 15 patients received prednisolone tablets per os; group 2 of 68 patients, intravenous methylprednisolone (MP) pulse therapy only; group 3 of 32 patients, intravenous MP pulse therapy plus vitamin D3; and group 4 of 40 patients, intravenous MP pulse therapy 8 to 12 months after thyroidectomy. Results: As soon as 3 months after treatment initiation, there was an improvement in condition of patients in all groups as assessed by clinical examination, followed by further improvement by 6 months and 12 months. The best results were obtained in patients of group 4, with a statistically significant improvement in clinical condition (p < 0.05). Retrobulbar adipose tissue thickness as assessed by orbital ultrasound at baseline and at 6 months and 12 months was statistically significantly greater in patients of all the four groups than controls (p < 0.05). At 6 months, serum TSHR-Ab levels in groups 1, 2 and 3 significantly decreased compared to baseline, with no significant difference between these groups, whereas serum TSHR-Ab level in group 4 was significantly higher than in other groups both at baseline and at 6 months. At 12 months, serum TSHR-Ab level in group 4 was significantly lower (р < 0.05) than in other groups (2.41 ± 0.81 mU/L versus 5.97 ± 1.71 mU/L for group 1, 5.49 ± 1.27 mU/L for group 2, and 6.17 ± 1.18 mU/L for group 3). Conclusion: Patients with EO associated with Graves’ disease in group 4 (intravenous MP pulse therapy after thyroidectomy) showed a significantly better (р < 0.05) treatment outcome than patients in other groups. Ultrasound imaging of retrobulbar adipose tissue thickness is inadequately informative for assessing treatment efficacy. Keywords: Graves’ disease, endocrine ophthalmopathy, TSHR-Ab, glucocorticoid pulse therapy, thyroidectomy, ultrasound imaging
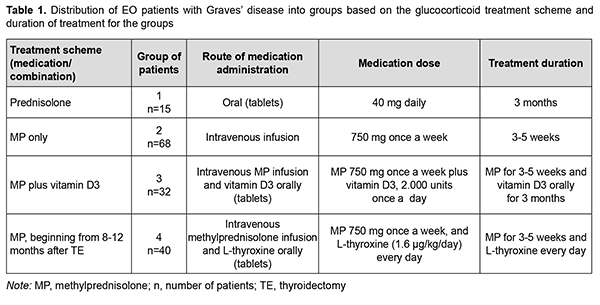
Introduction Endocrine ophthalmopathy (also referred to as Graves’ ophthalmopathy, endocrine orbitopathy, malignant exophthalmos, and thyrotoxic ophthalmopathy) is an organ-specific and progressive chronic autoimmune disease which is manifested by complex lesions of the soft tissues of the orbit, and characterized by edema, lymphocyte infiltration, proliferation of retrobulbar adipose tissue, extraocular muscles and adipose tissue, exophthalmos and ocular paresis [1, 2, 3, 4, 5, 6]. Autoimmune inflammatory process in retrobulbar soft tissue may develop in individuals with hypothyroidism, hyperthyroidism, or euthyroidism [7]. Endocrine ophthalmopathy (EO) is found in 5-20% of patients with Graves’ disease [8, 9, 10]. The prevalence of Graves’ orbitopathy (GO) in Europe is as high as about 10/10,000 persons, and the prevalence of unilateral GO is 0.50–1.50/10,000 persons [11]. The disease occurs more common in women than in men, and the incidence of Graves’ hyperthyroidism in the general population of Sweden is 210 per million per year, with a female:male ratio of 3.9: 1 and a peak incidence between 40 and 60 years. Of the people with the disease, almost 9 percent have a severe disorder, which can result in abrupt loss of vision due to optic neuropathy. The disease occurs more common in women than in men with a female:male ratio of 4:1, and a peak incidence between 40 and 60 years [12]. Ophthalmopathy is associated with Graves’ disease in 90-95% and with autoimmune thyroiditis in 5% of euthyroids or patients with hyperthyroidism. In addition, it is found in 5% of individuals in the presence of normal thyroid hormone and normal thyrotropin (TSH) levels and in the absence of history of autoimmune disease [5, 13, 14]. Management of endocrine ophthalmopathy associated with Graves’ disease is a current multidisciplinary issue. The treatment strategy depends on the severity of clinical symptoms and focuses first on the achieving and maintaining euthyroidism as soon as possible, and second on the subsequent management of EO proper [15]. Steroid therapy has been the first-line treatment of EO since 1960 [16, 17, 18]. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy (EGOGO) Guidelines for the Management of Graves' Orbitopathy recommend that high-dose intravenous glucocorticoids be considered as first-line treatment for moderate-to-severe and active GO [2, 11, 12]. Another treatment option is a combination of glucocorticoids with cytostatics and tyrosine kinase inhibitors [12, 19, 20]. The international guidelines state that glucocorticoid pulse therapy is the safest and most effective method of the introduction of steroid hormones. This treatment involves a single-stage high-dose introduction of steroid agent to cause immunosuppressive, anti-inflammatory and anti-edematous effects. The glucocorticoid pulse therapy frequency and dose depend on the severity of EO, patient’s age and presence or absence of chronic comorbidities. In addition, the choice of pulse therapy regimen depends on the clinical setting as well as patient fitness [2, 8, 21]. Methylprednisolone is currently the most popular drug used for prolonged treatment of patients with ophthalmopathy associated with active Graves’ disease [12, 22, 23]. In has been reported on the use of methylprednisolone in intravenous doses of 125 mg to 250 mg per day, with a subsequent change to a daily tablet dose of 1 to 1.5 mg/kg body weight. Others reported on the use of methylprednisolone pulse therapy at a dose of 500 mg per day once weekly for 6 weeks followed by of 250 mg per day once weekly for another 6 weeks. Some authors pointed to the evanescent effect of this treatment with a high risk of complications like liver insufficiency, elevated blood pressure, and abnormal metabolism of hydrocarbons [12, 22, 24, 25]. Treatment efficacy is assessed by clinical examination, orbital ultrasound imaging and/or orbital magnetic resonance imaging (MRI). Orbital ultrasound imaging is less accurate than orbital MRI with regard to the assessment of extraocular muscle volume, likely due to fibrosis which is common in EO and makes muscle delineation difficult [26, 27, 28]. In addition, MRI enables measurement of the thickest extraocular muscle compartment, whereas a viewing angle is a limitation of orbital ultrasound imaging [14]. Moreover, compared to MRI, orbital ultrasound imaging is less accurate for the visualization of the posterior orbit [28] and bone architecture, but it is more readily available, and, consequently, more frequently used for localization of the pathological process in EO. The purpose of this study was to assess the efficacy of various treatment regimens for patients with EO associated with Graves’ disease based on the retrospective analysis of clinical data, thyroid-stimulating hormone (TSH) receptor autoantibodies (TSHR-Ab) titers and orbital ultrasound imaging findings. Material and Methods We retrospectively reviewed the medical records of 155 patients with EO associated with Graves’ disease and either euthyroidism (in the presence of antithyroid therapy) or postoperative compensated hypothyroidism that underwent treatment at the Department of General Endocrine Pathology of the Komisarenko Institute for Endocrinology and Metabolism between 2009 and 2019. There were 125 women and 30 men (age, 18 years to 79 years; mean age, 49.68 ± 14.16 years). Disease duration ranged from 8 months to 36 months (median value, 27 months). Patients were divided into 4 groups based on the glucocorticoid treatment scheme. Group 1 of 15 patients received prednisolone tablets per os; group 2 of 68 patients, intravenous methylprednisolone (MP) pulse therapy; group 3 of 32 patients, intravenous MP pulse therapy plus vitamin D3; and group 4 of 40 patients, intravenous MP pulse therapy 8 to 12 months after thyroidectomy. In addition, to prevent the negative effects of glucocorticoid therapy (like hypopotassemia, edema and psychological arousal), patients received a potassium-based medication, sedatives and spironolactone (verospiron). Clinical data of patients with EO associated with Graves’ disease at baseline and 6 and 12 months after treatment initiation were reviewed. Measurements of serum TSH and FT4 were conducted at the laboratory of the Komisarenko Institute using chemiluminescent immunoassay kits (reference TSH range, 0.27-4.2 μIU/mL; reference FT4 range, 0.93-1.71 ng/div) on Cobas e 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Measurements of TSH-R-Ab were conducted using a chemiluminescence immunoassay (Immulite 2000 Xpi, Siemens Healthcare Diagnostics, Eschborn, Germany). Thyroid antibody titers were considered positive for the anti-TSH receptor-Ab titers above 0.55 U/L. These titers were determined for each group separately at baseline and 6 and 12 months after treatment initiation. Orbital ultrasonography was performed using NEMIO SSA-550A system (Toshiba Medical Systems Co, Ltd, Tokyo, Japan) and NEMIOXG SSA-580A system (Toshiba) equipped with 8–14 MHz linear probes to assess thickness of the retrobulbar orbital adipose tissue. Ultrasonography results for patients were compared with controls (a group of 20 healthy women aged 20- 64 years; mean age, 46.8 ± 4.1 years). The Student t test was used for statistical analyses. The level of significance p ≤ 0.05 was assumed. Data are presented as mean ± SEM. Pearson’s Chi-square (χ2) test with Yates’ Correction was used for comparison of clinical data among groups. For significance at the 0.05 level, the χ2 value had to be ≥3.84. Results Patients with Graves’ disease were medically treated for EO in the presence of stable euthyroidism, with a mean serum TSH of 1.12 ± 0.31 mU/L and mean fT4 of 1.43 ± 0.27 ng per scale division. In any patient, duration of EO did not exceed 12 months, with a mean duration of 9.2 ± 2.4 months for the study cohort. Clinical severity of EO was classified using the NOSPECS. Eighty-eight patients had class 3a, 3b, or 3c, and 67 patients, class 4a or 4b. All patients of the study had active EO (clinical activity score ≥3). Table 1 shows (a) the distribution of EO patients with Graves’ disease into groups based on the glucocorticoid treatment scheme and (b) duration of treatment for the groups. The retrospective analysis of medical records demonstrated that as soon as 3 months after initiation of treatment, there was a reduction in the number of patients having complaints, with 85 (54.84%) patients having no more complaints of tearing; 84 (54.19%) patients, no more complaints of itchy eyes; 99 (63.87%) patients, no more complaints of photophobia; 116 (74.84%) patients, no more complaints of spontaneous retrobulbar pain. At 6 months after initiation of treatment, only 5 (3.22%) patients had any of the above complaints, and all of these patients were from group 1 (treated with prednisolone per os). Patients from group 2 (MP pulse therapy only), group 3 (MP pulse therapy plus vitamin D3) and group 4 (MP pulse therapy after thyroidectomy) had not any of the above complaints at 6 months. In addition, eyelid edema was present in 7 patients at 6 months and in 3 patients at 12 months. All of these patients were from group 1. Moreover, a reduction in eyelid edema was observed in 25 (80.60%) patients from groups 2, 3 and 4 at 6 months after initiation of treatment.
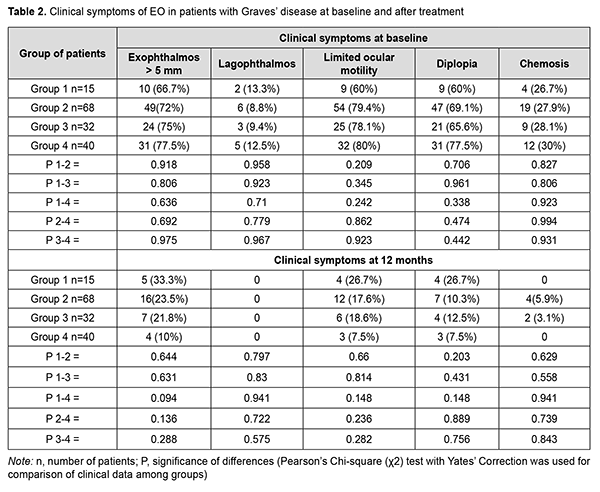
Clinical symptoms of EO in patients at baseline and after treatment are presented in Table 2.
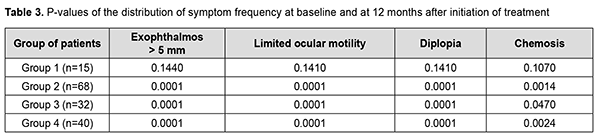
Table 3 shows the P-values of difference (Pearson’s Chi-square (χ2) test with Yates’ Correction) in exophthalmos exceeding 5 mm, limited ocular motility, diplopia and chemosis for each group at baseline and at 12 months after treatment initiation. Pearson’s Chi-square (χ2) test with Yates’ Correction was used for comparison of clinical data among groups. For significance at the 0.05 level, the χ2 value had to be ≥3.84.
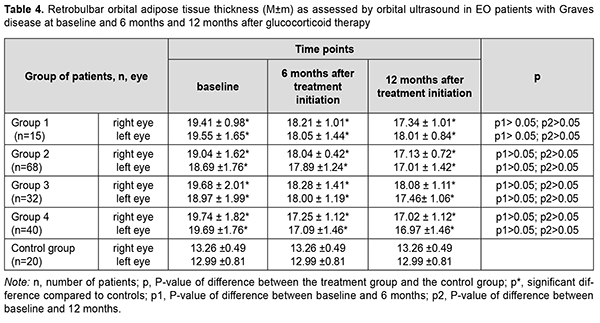
Statistically significant changes (improvements in exophthalmos, ocular motility, diplopia and chemosis) were noted in groups 2, 3 and 4 (Table 3). In addition, of the patients presenting with lagophthalmos at baseline, no patient had lagophthalmos after treatment (Table 2). In the course of treatment of EO with glucocorticosteroids, retrobulbar orbital adipose tissue thickness as assessed by ultrasound was expectedly greater in all the four groups than in the control group of healthy individuals (р < 0.05). In addition, at 6 months and at 12 months, no significant change in retrobulbar orbital adipose tissue thickness was observed in any treatment group compared to controls (р1 > 0.05) (Table 4).
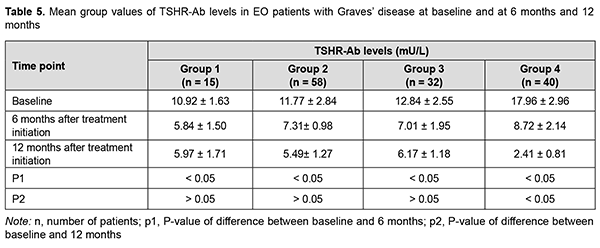
At 6 months, serum TSHR-Ab levels in groups 1, 2 and 3 significantly decreased compared to baseline, with no significant difference between these groups, whereas serum TSHR-Ab level in group 4 was significantly higher than in other groups (Table 5). At 12 months, there was no significant difference in serum TSHR-Ab levels in groups 1, 2 and 3 compared to 6 months, whereas serum TSHR-Ab level in group 4 was significantly lower (р < 0.05) than in other groups (2.41 ± 0.81 mU/L versus 5.97 ± 1.71 mU/L for group 1, 5.49 ± 1.27 mU/L for group 2, and 6.17 ± 1.18 mU/L for group 3).
Discussion Effective management of endocrine ophthalmopathy is a multidisciplinary concern, because the pathogenesis of this autoimmune disease is complex, and patients should be supervised by an ophthalmologist as well as an endocrinologist. The purpose of this study was to assess the efficacy of various treatment regimens for patients with both EO and Graves’ disease. In general, we found that glucocorticoid therapy was effective for treating EO, but intravenous glucocorticoid therapy was more effective than oral per os treatment, which was confirmed by clinical data. With treatment there was a decrease in the frequency of clinical symptoms like tearing, itching and spontaneous retrobulbar pain. In groups 2, 3 and 4 that received intravenous glucocorticoid treatment, there were improvements in exophthalmos and ocular motility, and a reduction in the number of patients experiencing diplopia and chemosis. In addition, of the patients presenting with lagophthalmos at baseline, no patient had lagophthalmos after treatment. Our findings are in agreement with those reported by Marcocci and colleagues [29] who demonstrated that intravenous administration was more advantageous than the oral route. Our analysis of changes in TSHR-Ab titers with glucocorticoid treatment demonstrated that in groups 1, 2 and 3 there was a significant decrease in TSHR-Ab titers until 6 months after treatment initiation, with no subsequent significant change at 12 months (Р3 > 0.05). In addition, TSHR-Ab level in group 4 was higher than in other groups at baseline, but it significantly decreased over the follow-up period (Р1,2 < 0.05), and was significantly lower than in other groups at 12 months (Р3 < 0.05). Therefore, findings of the current study are in agreement with our previous study [30], in which we observed a decrease in TSHR-Ab level and a gradual relief in the clinical symptoms of EO after surgical treatment for Graves’ disease [30]. We may state that post- thyroidectomy methylprednisolone therapy for EO was more effective due to the absence of the target for the production of TSHR antibodies and a faster decrease in the level of these antibodies over 12 months. This study also demonstrated some mismatch between the clinical data and the results of measurement of retrobulbar orbital adipose tissue thickness by orbital ultrasound. Obviously, orbital ultrasound is not the most reliable method for assessing the state of the eye over the course of therapy for EO, because it does not allow (a) a precise measurement of retrobulbar orbital adipose tissue thickness and (b) quantitative assessment of improvement in retrobulbar orbital adipose tissue edema during medical treatment of EO. MRI of the ocular muscles is the most informative method for monitoring the results of treatment of EO, allowing for (a) precise muscle thickness measurements and (b) assessment of reduction in edema volume during glucocorticoid therapy [28].
References 1.Taskina ES, Charinzeva SV, Charinzev VV, Serkin DM. [New opportunities in endocrine ophthalmopathy diagnostics (review)]. Klinicheskaya i eksperimental'naya tireoidologiya. 2017;13(3):20-28. Russian. 2.Taylor PN, Zhang L, Lee RWJ, Muller I, Ezra DG, Dayan CM, et al. New insights into the pathogenesis and nonsurgical management of Graves’ orbitopathy. Nat Rev Endocrinol. 2020 Feb;16(2):104-16. 3.Weiler DL. Thyroid eye disease: a review. Clin Exp Optom. 2017 Jan;100(1):20-25. 4.Khong JJ, McNab AA, Ebeling PR, Craig JE, Selva D. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol. 2016 Jan;100(1):142-50. 5.Dedov II, Melnichenko GA, Sviridenko NYu, Troshina EA, Fadeev VV, Belovalova IM, et al. [Federal clinical recommendations on diagnostics and treatment of endocrine ophthalmopathy associated with autoimmune thyroid pathology]. Problemy Endokrinologii. 2015;61(1):61-74. Russian. Crossref 6.Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, natural history, risk factors, and prevention of Graves' orbitopathy. Front Endocrinol (Lausanne). 2020 Nov 30;11:615993. 7.Kahaly GJ. Immunotherapies for thyroid eye disease. Curr Opin Endocrinol Diabetes Obes. 2019 Oct;26(5):250-5. 8.Olyinyk VA, Terekhovа GM, Buldygina YuV, Fed’ko TV, Klochkova VM, Rakov OV, Lysova ZG. Treatment of autoimmune ophthalmopathy in patients with diffuse toxic goiter by glucocorticoids. Endokrynolohiya. 2017; 22(2):108–14. 9.Novaes P, Diniz Grisolia AB, Smith TJ. Update on thyroid-associated Ophthalmopathy with a special emphasis on the ocular surface. Clin Diabetes Endocrinol. 2016 Nov 16;2:19. 10.Edmunds MR, Boelaert K. Knowledge of thyroid eye disease in Graves' disease patients with and without orbitopathy. Thyroid. 2019 Apr;29(4):557-62. 11.Perros P, Hegedüs L, Bartalena L, Marcocci C, Kahaly GJ, Baldeschi L, et al. Graves' orbitopathy as a rare disease in Europe: a European Group on Graves' Orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis. 2017 Apr 20;12(1):72. 12.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al; European Groupon Graves' Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J. 2016 Mar;5(1):9-26. Crossref PubMed 13.Pashkovska NV. [Endocrine ophthalmopathy in autoimmune diseases of the thyroid gland]. Mizhnarodnyi endokrynolohichnyi zhurnal. 2014;6 (62): 169–73. 14.Lanzolla G, Vannucchi G, Ionni I, Campi I, Sileo F, Lazzaroni E, Marinò M. Cholesterol serum levels and use of statins in Graves' orbitopathy: a new starting point for the therapy. Front Endocrinol (Lausanne). 2020 Jan 22;10:933. 15.Perros P, Dayan CM, Dickinson AJ, Ezra D, Estcourt S, Foley P, et al. Management of patients with Graves' orbitopathy: initial assessment, management outside specialised centers and referral pathways. Clin Med (Lond). 2015 Apr;15(2):173-8. 16.Strianese D. Efficacy and safety of immunosuppressive agents for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018 Jul/Aug;34(4S Suppl 1):S56-S59. 17.Hales IB, Thoma ID. Treatment of thyroid ophthalmopathy with corticoid analogues. Australas Ann Med. 1962 May;11:113-7. 18.Hodgson NM, Rajaii F. Current understanding of the progression and management of thyroid associated orbitopathy: a systematic review. Ophthalmol Ther. 2020 Mar;9(1):21-33. 19.Stan MN, Salvi M. Management of endocrine disease: Rituximab therapy for Graves' orbitopathy–lessons from randomized control trials. Eur J Endocrinol. 2017 Feb;176(2):R101-R109. 20.Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015 Feb;100(2):422-31. 21.Moleti M, Giuffrida G, Sturniolo G, Squadrito G, Campennì A, Morelli S, et al. Acute liver damage following intravenous glucocorticoid treatment for Graves' ophthalmopathy. Endocrine. 2016 Oct;54(1):259-68. 22.Nikonova LV, Tishkovskiy SV, Hadomskaya VI, Davydchyk EV, Doroshkevich IP. [Modern approaches to the therapy of endocrine ophthalmopathy]. Zhurnal Grodnenskogo meditsinskogo universiteta. 17(1):83-1. Russian. http://journal-grsmu.by/index.php/ojs/article/view/2371. 23.Riedl M, Kolbe E, Kampmann E, Krämer I, Kahaly GJ. Prospectively recorded and MedDRA-coded safety data of intravenous methylprednisolone therapy in Graves' orbitopathy. J Endocrinol Invest. 2015 Feb;38(2):177-82. 24.Covelli D, Vannucchi G, Campi I, Currò N, D'Ambrosio R, Maggioni M, Gianelli U, Beck-Peccoz P, Salvi M. Statins may increase the risk of liver dysfunction in patients treated with steroids for active graves' orbitopathy. J Clin Endocrinol Metab. 2015 May;100(5):1731-7. 25.Zoubek ME, Pinazo-Bandera J, Ortega-Alonso A, Hernández N, Crespo J, Contreras F, et al. Liver injury after methylprednisolone pulses: A disputable cause of hepatotoxicity. A case series and literature review. United European Gastroenterol J. 2019 Jul;7(6):825-837. 26.Kahaly GJ. Imaging in thyroid-associated orbitopathy. Eur J Endocrinol. 2001 Aug;145(2):107-18. 27.Yanik B, Conkbayir I, Acaroglu G, Hekimoglu B. Graves' ophthalmopathy: comparison of the Doppler sonography parameters with the clinical activity score. J Clin Ultrasound. 2005 Oct;33(8):375-80. 28.Nagy EV, Toth J, Kaldi I, Damjanovich J, Mezosi E, Lenkey A, et al. Graves' ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol. 2000 Jun;142(6):591-7. 29.Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell'Unto E, Rocchi R, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves' ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001 Aug;86(8):3562-7. 30.Buldygina YuV, Terekhova GM, Shlachtych SL, Fed’koTV, Klochkova VM, Strafun OS. [The results of surgical treatment of patients with diffuse toxic goiter and autoimmune ophthalmopathy]. Endokrynolohiya. 25(1): 5-10. Ukrainian.
Conflict of Interest Statement: The author states that there is no conflict of interest that could affect her opinion on the subject or materials of this manuscript. Sources of Support: none.
|