J.ophthalmol.(Ukraine).2022;3:3-9.
|
http://doi.org/10.31288/oftalmolzh2022339 Received: 17.01.2022; Accepted: 31.03.2022; Published on-line: 15.06.2022 Association of primary open-angle glaucoma with endothelial NO-synthase (NOS3) rs2070744 (T-786C) in a Ukrainian population O. A. Isaiev, V. M. Serdiuk Dnipro State Medical University; Dnipropetrovsk Regional Clinical Ophthalmology Hospital; Dnipro (Ukraine) TO CITE THIS ARTICLE:Isaiev OA, Serdiuk VM. Association of primary open-angle glaucoma with endothelial NO-synthase (NOS3) rs2070744 (T-786C) in a Ukrainian population. J.ophthalmol.(Ukraine).2022;3:3-9. http://doi.org/10.31288/oftalmolzh2022339
Background: Endothelial dysfunction and endothelial nitric oxide-synthase (NOS3) polymorphism play an important role in the pathogenesis of glaucomatous optic neuropathy (GON) in primary open-angle glaucoma (POAG). The rs2070744 (T-786C) variant is located in the promoter of the NOS3 gene and has been shown to be promising for association with POAG. Purpose: To identify an association between POAG and the rs2070744 (T-786C) variant in the NOS3 gene in a Ukrainian population. Material and Methods: We examined 200 patients, including 153 patients (153 eyes) diagnosed with POAG (the study group) and 47 patients (47 eyes) without POAG (the control group). The study group included patients with a mean age of 65.0 ± 13.1 years and disease duration of 4.9 ± 5.3 years. Genotypes were determined by real-time polymerase chain reaction (PCR). Mutation detection and quantification were performed using TaqMan Mutation Detection Assays (Life Technologies, Carlsbad, CA). Statistical analyses were performed using Statistica 10 software (StatSoft, Tulsa, OK, USA). Results: The minor T allele of rs2070744 was associated with a 1.7-fold decreased risk of POAG (OR = 0.589; 95% CI, 0.370-0.938; p = 0.025). The ancestral C allele of rs2070744 determined the genetic susceptibility of an individual to POAG. Patient carriers of the CC genotype of rs2070744 were 13.9 years and 13.2 years, respectively, younger (p < 0.001) than patient carriers of the СТ and ТТ genotypes. In addition, patient carriers of the C allele were younger than patient carriers of the T allele (61.9 ± 14.4 years versus 69.3 ± 9.6 years; p < 0.001). Eyes of patient carriers of the TT genotype showed better perimetric visual field mean deviation (MD) and pattern standard deviation (PSD), and thicker retinal nerve fiber layer (RNFL) and ganglion cell complex (GCC), compared to eyes of carriers of other genotypes. Conclusion: Allelic polymorphism rs2070744 in NOS3 had a protective effect against POAG, facilitating less severe manifestations and less severe progression of GON in a Ukrainian population. Keywords: primary open-angle glaucoma, rs2070744 (T-786C) NOS3.
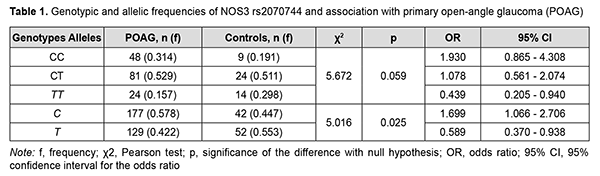
Introduction It is estimated that roughly 60.5 million people had glaucoma in 2010 and that this number is increasing [1]. In addition, it is estimated that, in 2019, 11.9 million people globally had moderate or severe vision impairment or blindness due to glaucoma, diabetic retinopathy and trachoma that could be prevented [2]. Wang and colleagues [3, 4] aimed to evaluate the trends and variations in global health burden of glaucoma by year, age and sex, region and socio-economic status, using disability-adjusted life years (DALYs). They found that, from 1990 to 2015, the DAILY number increased by 122%, and both male and female showed similar increasing trend with ageing, with the peak at 60 years old. In 2013, the number of people (aged 40-80 years) with glaucoma worldwide was estimated to be 64.3 million, increasing to 76.0 million in 2020 and 111.8 million in 2040 [5]. According to the American Optometric Association, primary open-angle glaucoma (POAG) develops slowly and usually without symptoms, and is the most common type of glaucoma [6]. The global prevalence of glaucoma for population aged 40-80 years is 3.54% (95% CI, 2.09-5.82) [7]. Black populations showed highest POAG prevalence, with 5.2% (95% credible interval (CrI) 3.7%, 7.2%) at 60 years, rising to 12.2% (95% CrI 8.9% to 16.6%) at 80 years. Increase in POAG prevalence per decade of age was greatest among Hispanics (2.31, 95% CrI 2.12, 2.52) and White populations (1.99, 95% CrI 1.86, 2.12) [8]. As early as 1994 Flammer described primary vascular dysregulation as a condition that transiently impairs adaptation of blood flow to the needs of the respective tissue [9]. Endothelial dysfunction plays a special role in the pathogenesis of glaucomatous optic neuropathy (GON; a specific damage to ganglious neurons and their axons) in POAG [10]. The endothelial vasoactive factors that can provoke vasoconstriction and optic nerve ischemia (nitric oxide (NO), endothelein, prostacyclin, etc.) play a role in the development of GON [10, 11]. The vascular endothelium, a monolayer of endothelial cells, constitutes the inner cellular lining of vessels, and regulates vascular tone and permeability, rheological properties of blood and homeostasis, proliferation of pericytes and vascular cells, fibrinolysis and inflammation [12, 13]. A functionally intact endothelium is characterized by its ability to secrete NO from L-arginine by the enzyme entodthelial NO synthase (eNOS; NOS3) [14]. NO is a lipophilic molecule produced at picomolar amounts, easily diffuses across cell membranes to myocytes, where it activates guanylate cyclase, producing cyclic guanosine monophosphate (cGMP) [15]. The latter acts as a messenger molecule in blood vessel dilation via the interaction with a specific protein kinase, which reduces the free Ca2+ level in myocytes [16]. It has been established that a significant proportion (20% to 60%) of cases of POAG are genetically determined [17, 18]. The NOS3 gene is located in chromosome 7q36.1. The rs2070744 (T-786C) variant is located in the promoter of the gene and among others has been shown to be promising for association with POAG [19]. The results of a study by Emam and colleagues (2014) [20] suggested that the CC genotype of T-786C NOS3 (rs2070744) may be associated with an increased risk of developing high-tension POAG in Egyptians, particularly, women. Kang and colleagues (2011) [21] showed that the association between hypertension and POAG depended on T-786C SNP variants, thus confirming the important role of vascular disease in the development of POAG. The purpose of this study was to identify an association between POAG and the rs2070744 (T-786C) variant in the NOS3 gene in a Ukrainian population. Material and Methods We examined 200 patients, including 153 patients (153 eyes) diagnosed with POAG (the study group) and 47 patients (47 eyes) without POAG (the control group). The study group included 57 men (37.25%) and 96 women (62.75%) with a mean age of 65.0 ± 13.1 years and disease duration of 4.9 ± 5.3 years, and the control group, 22 men (46.81%) and 25 women (53.19%), with no significant difference (р = 0.241) in gender between the groups. Patients underwent an examination according to the common protocol [3]; this included visual acuity testing, Humphrey perimetry, refractometry, intraocular pressure (IOP) measurement, biomicroscopy, gonioscopy, ophthalmoscopy, and ocular coherence tomography (OCT). In the study group, measurements in the affected eye were taken for the analysis and included best-corrected visual acuity (BCVA), IOP (mmHg), visual field mean deviation (MD; dB) and pattern standard deviation (PSD; dB), and OCT-derived peripapillary retinal nerve fiber layer (RNFL) thickness (µm), ganglion cell complex (GCC) thickness (µm), and Cup/Disc Area Ratio. In addition, a 3-ml venous blood sample was taken for genetic testing. Ethical approval was gained from the Ethics Committee of Dnipro State Medical University (protocol No. 6 of October 4, 2019). The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and the Order of the Ministry of Health of Ukraine No. 690 dated September 23, 2009, "On Approval of the Procedure for Conducting Clinical Trials of Medicinal Products and Expert Evaluation of Materials Pertinent to Clinical Trials and Model Regulations of the Ethics Committee". Genotypes were determined by real-time polymerase chain reaction (PCR) using Gene Amp® 7500 PCR System (Applied Biosystems, Foster City, CA). At the first phase of the study, DNA extraction from venous blood was done using the Invitrogen™ PureLink Genomic DNA Kit for Purification of Genomic DNA (Invitrogen Inc.) following the manufacturer’s instructions. Mutation detection and quantification were performed using TaqMan Mutation Detection Assays (Life Technologies, Carlsbad, CA). Statistical analyses were performed using Statistica 10 software (StatSoft, Tulsa, OK, USA). Data are presented as mean (M) and standard deviation (SD). The Fisher exact test and non-parametric Pearson chi-square test were applied for sample comparison. 3×2 and 2×2 chi-square test contingency tables were used to assess significance of differences in the frequencies of distributions of genotypes and alleles between the cases and controls. Odd ratios (OR) and 95% confidence intervals (CI) were calculated to assess association between alleles or genotypes with POAG. The level of significance p ≤ 0.05 was assumed. Results The data obtained regarding distributions of genotypes and alleles in the control group were compared with the data on the European population from the 1000 Genomes Project Phase 3 (http://www.internationa lgenome.org/). In general, the former data were consistent with the latter data. The frequency of the ancestral CC genotype of rs2070744 was 0.183 within the European population versus 0.191 within our Ukrainian population. The frequency of the heterozygous CT genotype was 0.511 within both populations. The frequency of the homozygous TT genotype was 0.306 in the European population compared with 0.298 in our Ukrainian population. The frequency of the ancestral C allele was 0.438 within the European population versus 0.447 within our Ukrainian population, and the frequency of the T allele, 0.562 versus 0.553, respectively. The above differences were not significant (p > 0.05). The frequency of the ancestral CC genotype of rs2070744 was higher (pFet = 0.139), whereas the frequency of the homozygous TT genotype lower (pFet = 0.049) in patients with POAG compared with controls (Table 1). In addition, the frequency of the ancestral C allele was higher, whereas the frequency of the T allele, lower, in patients with POAG compared with controls, and the differences were statistically significant (pFet = 0.033). Therefore, the frequencies of the minor genotype TT and allele T were statistically significantly (рFet < 0.05) increased in patients compared to controls.
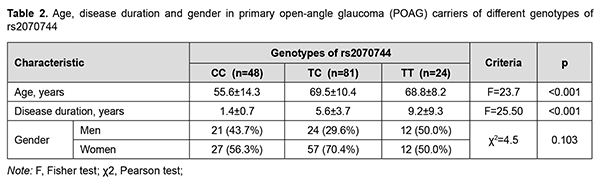
Testing for Hardy-Weinberg equilibrium demonstrated the random nature of inheritance in patients with POAG and in controls (χ2 = 1.119; p = 0.571 and χ2 = 0.051; p = 0.975, respectively). In addition, the association of distribution of genotypes of rs2070744 with the disease was not (p = 0.059), whereas the association of distribution of alleles of rs2070744 with the disease was statistically significant, and the ancestral C allele was associated with a 1.7-fold increased risk of POAG (OR = 1.699; 95% CI, 1.066-2.706; p = 0.025). Therefore, the ancestral C allele can determine genetic susceptibility to POAG in a Ukrainian population. The minor T allele of rs2070744 was associated with a 1.7-fold decreased risk of POAG (OR = 0.589; 95% CI, 0.370-0.938; p = 0.025), indicating a protective role of the NOS3 rs2070744 polymorphism in POAG. Given the above results, we analyzed the impact of rs2070744 on the phenotype of patients with POAG (Table 2). Compared to patient carriers of the CC genotype of rs2070744, patient carriers of the СТ and ТТ genotypes were 13.9 years and 13.2 years, respectively, older (p < 0.001), and had 7.8 years and 4.2 years, respectively, longer disease duration, and these differences were statistically significant (p < 0.001). It can be concluded that, in patients carriers of the high-risk ancestral CC genotype (rs2070744), the onset of the disease occurred at younger age, and disease course was more rapid. Therefore, rs2070744 polymorphism was associated with slower POAG course.
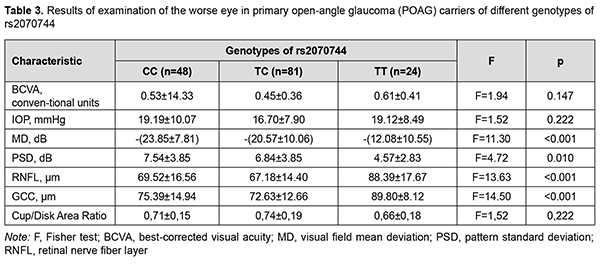
Compared to patient carriers of the ancestral C allele of rs2070744, patient carriers of the minor Т allele were 7.4 years older (p < 0.001; 69.3 ± 9.6 years versus 61.9 ± 14.4 years), and had 3.7 years longer disease duration (7.0 ± 6.6 years versus 3.3 ± 3.3 years; p < 0.001). There was no statistically significant difference (р = 0.103) between patients with POAG and controls regarding genotype frequency distribution stratified by gender. In addition, stratification by gender showed no association of SNP rs2070744 with POAG, with genotypic and allelic p values being р = 0.413 and р = 0.159, respectively, for men, and р = 0.122 and р=0.080, respectively, for women. There were certain differences among patient carriers of different genotypes with regard to eye examination results (Table 3). In general, patient carriers of the TT genotype exhibited a less severe disease course than carriers of other genotypes. Thus, eyes of patient carriers of the TT genotype showed 11.77 to -8.49 db (p<0.001) and 2.27 to 2.97 db (р = 0.010) better perimetric MD and PSD, respectively, and 18.87 to 21.21 µm (p < 0.001) and 14.41 to 17.17 µm (p < 0.001) thicker RNFL and GCC, respectively, compared to eyes of carriers of other genotypes.
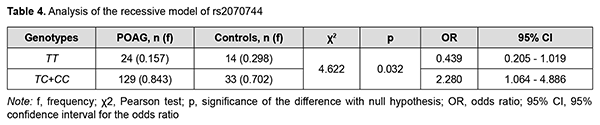
Carriers of the heterozygous genotype showed an intermediate position between carriers of the homozygous genotypes with regard to eye examination results, which in our opinion suggested a pathogenetical role of the C allele of rs2070744. This was confirmed by the inheritance model analysis which demonstrated the significance of the recessive model (СС+СТ vs ТТ; р = 0.032), but not the dominant model (СС vs СТ+ТТ; р = 0.105). In addition, it was demonstrated in the recessive model (Table 4) that patient carriers of the ancestral C allele (СС+СТ genotypes) are at a higher risk of POAG compared to patient carriers of the homozygous TT genotype (р = 0.032), whereas carriers of the minor homozygous TT genotype are at a 2.3-fold lower risk compared to carriers of the СС and СТ genotypes (р = 0.032).
Therefore, the current study demonstrated genetic susceptibility to POAG in patient carriers of the ancestral C allele of rs2070744, with a 1.7-fold increased risk of the disease (OR = 1.699; 95% CI, 1.066-2.706; p = 0.025). In addition, in these patients, the onset of the disease occurred at younger age, disease course was more rapid, perimetric characteristics were worse, and retinal layer degeneration was more apparent, indicating a more active development of GON compared to patient carriers of other genotypes. Moreover, it could be established that allelic polymorphism rs2070744 in NOS3 had a protective effect against POAG. Discussion Others have reported on the association of the rs2070744 NOS3 SNP with POAG in other populations. In an Emam and colleagues’ study [20] including 160 Egyptean patients (76 men and 84 women; age range, 41-75 years) with POAG, the CC genotype was significantly associated with POAG (OR = 2.54, 95% CI = 1.26-5.13, p = 0.007) and the C allele was significantly associated with POAG (OR = 1.86, 95% CI = 1.29-2.69, p < 0.001). Da Silva and colleagues [22] found that in a Brazilian cohort of patients with POAG, the T-786C NOS3 polymorphism was significantly associated as a risk factor for POAG among women. Kondcar and colleagues [23] investigated the association of NOS3 gene polymorphisms in patients with POAG (94 men and 79 women) of Saudi origin, and found that haplotypes CG and CT of rs2070744 and rs1799983 can significantly modulate the risk of POAG, particularly among men only. Xiang and colleagues (2016) [24] conducted a meta-analysis to clarify the association of eNOS polymorphisms and POAG. Their overall results showed that both TT genotype in rs2070744 and GG genotype in rs1799983 are associated with decreased risk of POAG susceptibility. Stratified analysis based on ethnicity showed that the association of rs2070744 with POAG remained only in Caucasians. The latter fact could explain the finding of no association for individuals of Asian origin [23] and was in agreement with our findings. There is no agreement in the literature regarding gender differences for the association between NOS3 polymorphisms and POAG. Most studies, including meta-analyses of recent years, have reported on the association between NOS3 polymorphisms and POAG for women, but not for men [20, 22, 24]. In a study by Emam and colleagues [20], after stratification by gender, the CC genotype and the C allele were significantly associated with POAG in women only (OR = 3.06, 95% CI = 1.07-8.74, p = 0.03 for the CC genotype, and OR = 2.09, 95% CI = 1.24-3.53, p = 0.005 for the C allele). Kang and colleagues [25] demonstrated that NOS3 genotype - female reproductive health interactions are important in POAG pathogenesis. Among the women who were homozygous for the common allele of the T −786C SNP, current postmenopausal hormone use was significantly inversely associated with high-tension POAG risk (multivariate RR = 0.41; 95% CI, 0.22–0.76) [26]. In addition, the association between hypertension and POAG depended on T-786C SNP variants. Compared with TT homozygotes without hypertension, the TT homozygotes with hypertension were at significantly higher risk (RR=1.45, 95% CI = 1.01, 2.08); however, among carriers of the variant (C) allele hypertension was not associated, or even showed protective associations (p-interaction = 0.007) [21]. This suggested an association of the rs2070744 NOS3 SNP with such a vascular disease as hypertension, and could cause an association between the latter and POAG [27]. In the current study, after stratification by gender, no significant association was found between rs2070744 POAG; however, there was a tendency for the association for women (р = 0.080), which prompted for further studies and patient stratification by reproductive status and by the presence of hypertension. In a recent review [19] of 9 studies on polymorphism rs2070744 with a sample size of 1631 subjects and a control group of 2405 subjects related to polymorphism rs2070744, it was shown that rs2070744 may increase the risk of POAG among individuals. Therefore, in the current study, a protective role of the NOS3 rs2070744 polymorphism in POAG was demonstrated. In addition, it can be seen that this effect is more substantial among women, which depends on reproductive status and the presence of hypertension. The above raises the possibility that rs2070744 polymorphism exerts an effect on the onset of POAG through the effect of rs2070744 on the development of endothelial dysfunction, with women carriers of the ancestral C allele being at an increased risk of POAG, with the risk being especially high in CC homozygotous women, particularly those of menopausal age. In the current study, the role of the NOS3 rs2070744 polymorphism in POAG was confirmed by better perimetric MD and PSD in eyes of patient carriers of the TT genotype compared to eyes of patient carriers of other genotypes. Conclusion First, the NOS3 rs2070744 polymorphism had an association with POAG in a Ukrainian population, with the minor T allele of rs2070744 being associated with a 1.7-fold decreased risk of POAG (OR = 0.589; 95% CI, 0.370-0.938; p = 0.025), indicating a protective role of the polymorphism in POAG. The ancestral C allele of rs2070744 determines the genetic susceptibility of an individual to POAG. Second, carriers of the minor T allele of rs2070744 who developed POAG were found to be older than carriers of the C allele who developed the disease (p < 0.001). Finally, eyes of patient carriers of the TT genotype showed better perimetric MD and PSD, and thicker RNFL and GCC, respectively, compared to eyes of carriers of other genotypes.
References 1.Ling JD, Bell NP. Role of cataract surgery in the management of glaucoma. Int Ophthalmol Clin. 2018;58(3):87-100. 2.Cieza A, Keel S, Kocur I, Mccoy M, Mariotti SP. World report on vision. Geneva: World Health Organization; 2019. [Internet]. Available from: https://www.who.int/health-topics/blindness-and-vision-loss#tab=tab_1. 3.Wang W, He M, Li Z, Huang W. Epidemiological variations and trends in health burden of glaucoma worldwide. Acta Ophthalmol. 2019;97(3):e349-e355. 4.Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV et alt. Vision loss expert group of the global burden of disease study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e34. 5.Tham, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90. 6.Glaucoma. American Optometric Association [Internet]. Available from: https://www.aoa.org/healthy-eyes/eye-and-vision-conditions/glaucoma?sso=y. 7.Agrawal P, Bradshaw SE. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (MIGS) in primary open-angle glaucoma. Ophthalmol Ther. 2018;7(1):49-73. 8.Kapetanakis VV, Chan MP, Foster PJ, Cook DG, Owen CG, Rudnicka AR. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100(1):86-93. 9.Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38 Suppl:S3-6. 10.Mudassar IB, Yew KK, Thambiraja R, Sulong S, Ghulam RA, Ahmad TL. Microvascular endothelial function and primary open angle glaucoma. Ther Adv Ophthalmol. 2019;11:2515841419868100. 11.Hoguet A, Chen PP, Junk AK, Mruthyunjaya P, Nouri-Mahdavi K, Radhakrishnan S, Takusagawa HL, Chen TC. The Effect of Anti-Vascular Endothelial Growth Factor Agents on Intraocular Pressure and Glaucoma: A Report by the American Academy of Ophthalmology. Ophthalmology. 2019 Apr;126(4):611-622. 12.Vargas-Valderrama A, Messina A, Mitjavila-Garcia MT, Guenou H. The endothelium, a key actor in organ development and hPSC-derived organoid vascularization. J Biomed Sci. 2020;27(1):67. 13.Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. 2019;20(18):4411. 14.Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit Care Clin. 2020;36(2):307-321. 15.Godo S, Shimokawa H. Endothelial functions. Arterioscler Thromb Vasc Biol. 2017;37(9):e108-e14. 16.Wettschureck N, Strilic B, Offermanns S. Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev. 2019;99(3):1467-1525. 17.Efendieva MH, Budzinskaya MV, Kadyshev VV, Zinchenko RA, Savochkina OA, Pupysheva AD. [Molecular and genetic aspects of age-related macular degeneration and glaucoma]. Vestn Oftalmol. 2019;135(3):121-127. Russian. 18.Liu Y, Allingham RR. Major review: Molecular genetics of primary open-angle glaucoma. Exp Eye Res. 2017;160:62-84. 19.Salari N, Bokaee S, Farshchian N, Mohammadi M, Kazeminia M. The role of polymorphisms rs2070744 and rs1799983 eNOS gene in patients with POAG: a systematic review and meta-analysis. Int Ophthalmol. 2021 Apr 10. https://doi.org/10.1007/s10792-021-01832-y. Epub ahead of print. PMID: 33837898. 20.Emam WA, Zidan HE, Abdulhalim BE, Dabour SA, Ghali MA, Kamal AT. Endothelial nitric oxide synthase polymorphisms and susceptibility to high-tension primary open-angle glaucoma in an Egyptian cohort. Mol Vis. 2014;20:804-11. PMID: 24940036; PMCID: PMC4057245. 21.Kang JH, Wiggs JL, Rosner BA, Haines J, Abdrabou W, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with hypertension, alcohol intake, and cigarette smoking. Arch Ophthalmol. 2011 Jun;129(6):773-80. 22.Magalhães da Silva T, Rocha AV, Lacchini R, Marques CR, Silva ES, Tanus-Santos JE, Rios-Santos F. Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk of primary open angle glaucoma in a Brazilian population. Gene. 2012 Jul 10;502(2):142-6. 23.Kondkar AA, Azad TA, Sultan T, Osman EA, Almobarak FA, Al-Obeidan SA. Association of endothelial nitric oxide synthase (NOS3) gene polymorphisms with primary open-angle glaucoma in a Saudi cohort. PLoS ONE. 2020;15(1): e0227417. 24.Xiang Y, Dong Y, Li X, Tang X. Association of Common Variants in eNOS Gene with Primary Open Angle Glaucoma: A Meta-Analysis. J Ophthalmol. 2016;2016:1348347. 25.Kang JH, Wiggs JL, Haines J, Abdrabou W, Pasquale LR. Reproductive factors and NOS3 variant interactions in primary open-angle glaucoma. Mol Vis. 2011;17:2544-51. Epub 2011 Sep 30. PMID: 22025889; PMCID: PMC3198482. 26.Kang JH, Wiggs JL, Rosner BA, Hankinson SE, Abdrabou W, Fan BJ, Haines J, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010 Feb;51(2):971-9. 27.McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10(2):71-8.
Disclosures Corresponding author: O.A. Isaiev, email: oleksiiisaev@gmail.com Disclosures: The authors state that there is no conflict of interest in the preparation of this article. Source(s) of support: There are no external sources of funding. Abbreviations: NOS3, nitric oxide-synthase; GON, glaucomatous optic neuropathy; GON, glaucomatous optic neuropathy; POAG, primary open-angle glaucoma; PCR, polymerase chain reaction; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; GCC, ganglion cell complex; CI, confidence interval; CrI, credible interval; eNOS, NOS3, the enzyme entodthelial NO synthase; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; IOP, intraocular pressure; OCT, ocular coherence tomography; M, mean; SD, standard deviation; OR, Odd ratios.
|