J.ophthalmol.(Ukraine).2022;5:65-70.
|
http://doi.org/10.31288/oftalmolzh202256570 Received: 15.07.2022; Accepted: 14.09.2022; Published on-line: 27.10.2022 Non-Hodgkin B-cell MALT lymphoma of marginal zone of the upper eyelid N. V. Malachkova 1, Ye. L. Prus 2, S. O. Mahdebura 3 1 National Pirogov Memorial Medical University, Vinnytsya 2 Optimal-M LLC 3 Podilsky regional center of oncology Vinnytsia (Ukraine) TO CITE THIS ARTICLE: Malachkova NV, Prus YeL, Mahdebura SO. Non-Hodgkin B-cell MALT lymphoma of marginal zone of the upper eyelid. J.ophthalmol.(Ukraine).2022;5:65-70. http://doi.org/10.31288/oftalmolzh202256570
Background: Non-Hodgkin lymphoma (NHL) represents a heterogenous group of malignant lymphoproliferative neoplasms originating from lymphoid tissue cells and having distinct clinical, cytomorphological, immunological and molecular and genetic features. Primary orbital lymphomas are rare and account for approximately 1% of all NHL. Lymphomas are, however, the most common primary orbital tumor in adults 60 years of age and older. Purpose: To present a rare case of primary B-cell MALT lymphoma of marginal zone of the upper eyelid, to highlight its clinical features, and to demonstrate the importance of a multidisciplinary approach to its treatment. Results: A male patient presented to the eye clinic complaining of bilateral pterygium and periodic nasal congestion. Within the course of examination and prolonged observation, the ophthalmologist suspected the symptoms of massive orbital disease, and the patient was referred for magnetic resonance imaging (MRI) of the brain. On the basis of imaging assessment and the oncologist’s opinion, the patient was hospitalized at the Podillia Regional Oncology Center with a preliminary diagnosis of orbital lymphoma. Thereat, a final diagnosis of “extranodal, non-Hodgkin’s B-cell lymphoma of the marginal zone (MALT lymphoma) of Stage 3a, with metastases in the lungs, pleura and lymph nodes of the mediastinum” was established. At the moment, he is under observation by an oncologist and on the way to stabilization of his major disease. Conclusion: Management of orbital non-Hodgkin’s lymphomas remains a challenge requiring a multidisciplinary approach involving ophthalmologists and oncologists. Keywords: orbit, non-Hodgkin’s lymphoma, oncologist, brain, biopsy
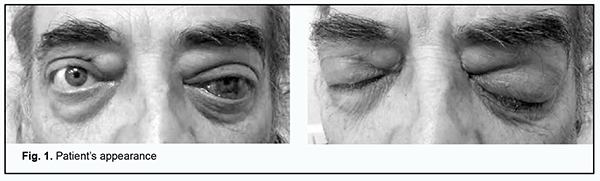
Introduction Lymphoma is a cancer of the lymphatic tissue which is characterized by enlargement of the lymph nodes and/or lesions of various internal organs, with uncontrolled accumulation of oncogenic lymphocytes therein [1]. Non-Hodgkin lymphoma (NHL) represents a heterogenous group of malignant lymphoproliferative neoplasms originating from lymphoid tissue cells and having distinct clinical, cytomorphological, immunological and molecular and genetic features. Primary orbital lymphomas account for approximately 1% of all NHL [2]. Lymphomas are, however, the most common primary orbital tumor in adults 60 years of age and older [3]. Most NHL of the orbit and orbital adipose tissue are extranodal B-cell lymphomas of the marginal zone which are mucosa associated lymphoid tissue (MALT) lymphomas. Because there are more than 80 types of NHL, the diagnostic assessment is difficult [4]. The first reports on MALT gastric lymphoma have been associated with Helicobacter pylori (H. pylori) infection. There have subsequently appeared reports on MALT lymphoma occurring in other epithelial structures (like thyroid gland, aural gland, lungs, mammary gland) and the orbit [5]. Non-Hodgkin lymphoma originates from abnormal B-cells or T-cells. Most (more than 80%) cases of non-Hodgkin lymphoma are associated with impaired life cycle of B cells. Diffuse large B cell lymphoma is the most common lymphoma (30.6%), followed by follicular lymphoma (22.1%), whereas B-cell lymphoma of the marginal zone, mantle cell lymphoma, and Burkitt lymphoma are less common. T-cell lymphomas (e.g., peripheral T-cell lymphomas and cutaneous T-cell lymphomas) account for less than 20% of all the non-Hodgkin lymphomas. Treatment strategy is determined by the origin of the disease [6]. The most significant factor for the development of NHL is age. The NHL prevalence in 15-20 year olds was estimated to be ten times lower than in individuals older than 75 years of age, and this is true for both genders. Most patients with NHL are older than 60 years [6]. Malignant ocular lymphoma (NHL of the orbit, eye and ocular adnexa) accounts for 2-4% of all the extranodal malignant lymphomas [7]. In addition, malignant lymphoma of the orbit accounts for more than 40% of all malignant neoplasms of the orbit, and primary malignant lymphoma is more common than secondary malignant lymphoma. This requires increased attention of ophthalmologists [8]. Coupland and colleagues [9] retrospectively reviewed medical records of 112 patients with ocular adnexal lymphoproliferative lesions and believed that the prognosis of the disease depends only on spread of the disease before the onset of ophthalmic symptoms, i.e., on the Ann Arbor stage. Some authors are of the opinion that, in the presence of ocular lesions, it is the morphological and immunological subtype of lymphoma that determines the prognosis of the disease [10,11]. Others found that the single most important and statistically significant prognostic factor in these patients was the extent of disease at the time of presentation with an ocular adnexal lymphoid proliferation [12]. For example, the prognosis for patients presenting with conjunctival lymphoid proliferations is more favorable than for those presenting with eyelid lymphoid proliferations [13]. Khalil and colleagues [14] believe that generalized lymphoma is common in conjunctival non-Hodgkin’s lymphomas. [14]. The prognosis for life for patients presenting with intraocular lymphoma is unfavorable [15,16,17,18]. Medeirоs compared ocular adnexal lymphomas of various sites and revealed no significant difference in risk of dissemination [19]. At the International Congress of Ocular Oncology in 2009 it was proposed to consider bilateral lesions as an unfavorable prognostic factor. In most patients, clinical signs of orbital lymphoma are characterized by a gradual increase in the severity of symptoms, and depend on the site of the tumor. For example, when the lesion is attached to the orbital wall, exophthalmos develops with contralateral displacement of the globe; when the tumor lies in the muscle funnel, axial exophthalmos develops. As the tumor grows, the exophthalmos increases, reposition of the globe becomes more difficult, ocular motility becomes limited, and diplopia appears. The exophthalmos is usually moderate (less than 5 mm) and painless and increases gradually. Sometimes the process is accompanied by an increased intraocular pressure (IOP) leading to conjunctival injection [8]. Orbital lymphoma should be differentiated from endocrine ophthalmopathy, retrobulbar adipose tissue tumor, Parinaud's syndrome, cavernous sinus syndrome, cavernous hemangioma, carotid cavernous fistula, parasitic infection of the orbital soft tissues, and other bulky or massive orbital neoplasms (like optic nerve sheath meningioma, local neurofibroma, orbital dermoid cyct, neurofibromatosis, fibrous histiocytoma of the orbit, etc.) and brain neoplasms. At magnetic resonance imaging (MRI), orbital lymphomas commonly appear as unifocal or multifocal homogeneous masses with sharp margins. Isolated lesions of the extraocular muscles are very rare. The treatment strategy depends on the histological type and clinical stage of lymphoma; presence of the prognostic factors at the onset of the disease; functional state of the patient; and presence of comorbidities. Most patients with indolent lymphomas present with a far advanced (Ann Arbor stage 3 or 4) neoplastic process, which is incurable. In patients presenting with a limited (Ann Arbor stage 1 or 2) neoplastic process, spontaneous regression of the disease sometimes occurs, or the disease may be cured through the eradication of the etiological factor and/or surgical excision of the primary focus of the lymphoma [20]. Prognosis depends on the subtype of lymphoma. More than 50% of patients with indolent lymphomas exhibit remission, which is, however, not prolonged (not longer than several years), and recovery is sporadic. Of patients with aggressive lymphomas, more than 60% exhibit remission, and 40–50% exhibit recovery. The five-year survival rate for patients with NHL varies, depends on the morphology of the tumor, and is above 70% for patients with B-cell lymphomas of the marginal zone or follicular lymphomas and is lower than 30% for those with T-cell lymphoblastic lymphomas or peripheral T-cell NHL [21]. The purpose of this paper was three-fold: (1) to present a rare case of primary B-cell MALT lymphoma of marginal zone of the upper eyelid, (2) to highlight its clinical features, and (3) to demonstrate the importance of a multidisciplinary approach to its treatment. Case report A male patient, born in 1964, presented to the ophthalmologist and complained of a wing-shaped film-like conjunctival mass blocking the left pupil and another similar but smaller mass in the right eye. In addition, the patient complained of periodic nasal congestion and difficulties with nasal breathing. Anamnesis vitae: He denied any family history of hereditary or genetic disorders and any history of surgery. He also denied any history of tuberculosis, hepatitis A or sexually transmitted disease. The allergic history was negative. Anamnesis morbi: The wing-shaped masses on the conjunctiva of both eyes appeared about 18 months before presentation and slowly increased in area with time. The patient associated them with a several years’ history of a bilateral eye injury due to a chemical burn. The details of the origin of the chemical were unavailable. Although the wing-shaped masses on the conjunctiva had increased with time, the patient had not sought medical care for his symptoms. He reported that he attended the ophthalmologist because the film-like mass blocking the left pupil made it impossible to fully see with the left eye. On examination, the face was massive and showed hypertelorism. The patient was found to have bilateral exophthalmos, more severe on the right, and a bilateral upper eyelid mass, larger on the right, like retrobulbar adipose tissue hernia, felt dense on palpation, but not fused to the surrounding tissues or bones. When asked how long ago he noted the protrusion of his eyes and tumor-like masses above them, the patient answered that he had always looked like that. The patient had bilateral pterygium. His uncorrected and best-corrected visual acuity (with a spherical correction of +2.75 D) was 0.3 OD and 0.8 OD, respectively. Eccentric visual acuity was 0.1 OS. Sphere, cylinder and axis values measured by noncyploplegic autorefractometry were 3.0 D, 0.25 D and 165°, respectively, OD. We failed to perform autorefractometry in the left eye due to the pterygium. Intraocular pressure (IOP) was 23.0 mmHg OD (not measured OS due to the conjunctival neoplasm). Slit-lamp biomicroscopy OD showed pale pink palpebral and bulbar conjunctivae; pterygium of grades 1-2; a clear, smooth and bright cornea; a moderately deep anterior chamber; a normal iris; a clear lens; and filamentous vitreous opacities. Slit-lamp biomicroscopy OS showed a pale pink bulbar conjunctiva and pterygium of grades 3-4. Fundus examination OD showed a pale pink optic disc with distinct margins. Arterial to vein ratio was 1/3. The right macular site was normal. A fundus examination was not performed in the left eye because of the pterygium. The patient was diagnosed with pterygium grades 1-2 OD and pterygium grades 3-4 OS; hypertonic retinal angiopathy, and possible bilateral retrobulbar adipose tissue herniation. He was recommended to consult an otolaryngologist to clarify the condition of the paranasal sinuses. The decision was made to perform surgery for microsurgical removal of pterygium of grades 3-4 and to monitor the patient’s retrobulbar adipose tissue hernia thereafter. Surgical treatment of the left pterygium was performed, and the patient was discharged with recommendations for bimonthly outpatient follow up for evaluation of adipose tissue herniation. He revisited the clinic after failing to attend for outpatient follow-up visits within six months of being discharged. The patient noted the protrusion of his eyes and that his bilateral upper eyelid tumor had progressed. In addition, he reported that within 6 months after being discharged, he visited his otolaryngologist several times for difficulties with periodic nasal breathing, was treated for maxillary sinusitis and exhibited periodic swelling of the soft tissues in the projection of the frontal sinuses. The otolaryngologist recommended consulting an ophthalmologist repeatedly. The patient believed that he would benefit from receiving a cosmetic blepharoplasty because he was sure that his swelling and neoplasm masses at the site of eyelids were due to age-related skin changes. On re-examination, the patient was found to have bilateral exophthalmos, more severe on the right, which had increased after the initial examination; exodeviation of the right eye of 15 degrees, based on the Hirschberg test; a 2x2 cm tumor-like protruding mass at the site of the right upper eyelid and a subcutaneous roller-shaped upper eyelid mass spread over the surface of the right upper eyelid and felt dense on palpation. At the left, there was a 2x1.5 cm diffuse roller-shaped upper-eyelid mass, felt somewhat firm to the touch. In addition, there were similar but significantly smaller masses on the lower eyelids of both eyes. Figure 1A, B shows the patient’s appearance.
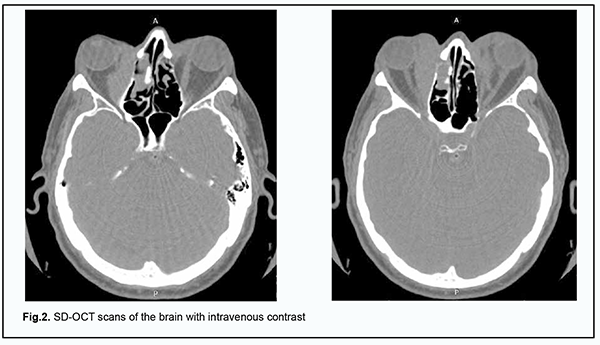
He was recommended to have a brain MRI with a focus on bilateral orbital imaging; spectral-domain optical coherence tomography (SD-OCT); and a complete blood cell (CBC) count. In addition, he was recommended to consult an endocrinologist and an oncologist. CBC results: hemoglobin (Hgb), 115 g/L; red blood cells (RBC), 4.2 х 106/mm3; white blood cells (WBC), 6.0 х 103/mm3; platelets, 2.4 х 105/mm3; percentage of bands, 3%; percentage of segmented neutrophils, 3%; percentage of eosinophils, 5%; percentage of eosinophils, 2%; percentage of lymphocytes, 25%, and RBC sedimentation rate, 17 mm/h. Blood: AB+ Rh positive. Blood biochemistry analysis: total blood protein, 74.6 g/L; urea, 6.4 mmol/L; creatinine, 88.0 µmol/L; total bilirubin, 21.6 µmol/L. Thyroid analysis: thyroid-stimulating hormone (TSH), 1.10 µU/ml; free thyroxine (FT4), 1.0 ng/div; anti-thyroid peroxidase antibodies, 0.9 U/ml. It was concluded that the patient had mild anemia and mild hyperbilirubinemia. On May 8, 2018, he underwent brain MRI at the Radiographic Imaging Department of the Vinnytsia Regional Clinical Hospital. Based on the results of MRI, the conclusion was as follows: Additional masses of unclear origin were noted in the superior and medial orbital cones. One cannot exclude changes in the muscles in the presence of changes in the thyroid gland and inflammation. It is likely that the neoplasms are in the presence of fibromatosis. Figure 2A, B shows SD-OCT scans of the brain with intravenous contrast.
Biopsy was taken from the upper eyelid neoplasms by the oncologist in accordance with MRI-based and SD-OCT-based conclusions. Biopsy analysis demonstrated that the tumor cell morphology and the expression of antibodies by tumor cells corresponded to an extranodal B-cell lymphoma of the marginal zone (MALT lymphoma). Because of biopsy results, on May 29, 2018, the patient was hospitalized at the Podillia Regional Oncology Center with a preliminary diagnosis of orbital lymphoma. On January 1, 2019, computed tomography of the brain, chest and abdomen with intravenous contrast was performed, demonstrating reduced pneumatization of the left frontal sinus with a contrasted soft tissue component at the level of paranasal sinuses; apneumatosis of the right medial ethmoid labyrinth; and fluid with small air inclusions at the level of the right maxillary sinus. In addition, CT demonstrated a 32 x 14 mm elongated soft-tissue mass in the right medial orbit displacing the medial rectus laterally and a similar 25 x 13 mm mass in the left proximal orbit. It was concluded that there was CT evidence of a right maxillary sinusitis, ethmoiditis, focal changes in the left frontal sinus, orbits, lungs, and posterior mediastinum, likely as manifestations of the primary disease, a non-Hodgkin's lymphoma. On April 2, 2019, computed tomography of the brain and abdomen without intravenous contrast was performed, and it was concluded that there was no CT evidence of focal volume changes in the brain, but there was CT evidence of polysinusitis progression and regression of focal changes in the orbit and lungs. The patient was consulted by a department therapist. Therapist's opinion: coronary artery disease (atherosclerotic cardiosclerosis, heart failure 0 level), chronic pancreatitis in unstable remission, and osteochondrosis of the cervical spine. On June 15, 2019, multislice spiral computed tomography (MSCT) of the chest and abdomen with intravenous contrast was performed, demonstrating signs of secondary lesions of the lungs, pleura and lymph nodes of the mediastinum. The patient underwent inpatient surgery for removal of a soft tissue tumor of the right upper eyelid. Gross specimen description: The tumor, 2.0 cm in diameter, was round, hard and knobbly. The tumor when excised showed a thin wall and homogeneous structure, was pale pink and hard at touch. Histopathological conclusion: The tumor exhibited morphological findings and the pattern of antibody expression strongly suggestive of extranodal, non-Hodgkin’s B-cell lymphoma of the marginal zone (MALT lymphoma). Given the results of primary and adjunct methods of examination, a final diagnosis of “extranodal, non-Hodgkin’s B-cell lymphoma of the marginal zone (MALT lymphoma) of stage 3a, with stage 3 metastases in the lungs, pleura and lymph nodes of the mediastinum” was established. The secondary diagnosis was coronary artery disease (atherosclerotic cardiosclerosis, heart failure 0 level), chronic pancreatitis in unstable remission, and osteochondrosis of the cervical spine. A decision was made to administer a course of polychemotherapy. On March 11, 2019, the patient was re-admitted to the Podillia Regional Oncology Center (radiotherapy) for in-patient treatment with a diagnosis of “non-Hodgkin’s B-cell lymphoma of stage 3 and Clinical Group 3; coronary artery disease (atherosclerotic cardiosclerosis, heart failure 0 level) and chronic pancreatitis in remission”. He received brain radiotherapy (32 Gy) and 8 courses of polychemotherapy, and was discharged with recommendations for rehabilitation. The patient was followed up at the local otolaryngologist’s office and at the oncologist’s office at the Podillia Regional Oncology Center, Vinnytsia, and was recommended to have CT of the paranasal sinuses. Repeat CT of the paranasal sinuses performed on August 9, 2019, showed mild improvement (a reduced soft-tissue component at the level of the paranasal sinuses). In addition, the CT examination revealed no change in the bone defects of the medial wall of the right orbit. No visible pathological changes in the brain were observed. The patient was observed by the ophthalmologist throughout the course of treatment and attended follow-up appointments. He is stabilized at present with regard to ocular symptoms, has no ocular complaints. In addition, visible regression of eyelid tumors and exophthalmos is observed, and the position of the eyes is almost correct. At the moment, the patient is under observation by an oncologist and on the way to stabilization of his major disease. Discussion Management of orbital non-Hodgkin’s lymphomas remains a challenge requiring a multidisciplinary approach involving ophthalmologists and oncologists. The ophthalmologist is to detect the orbital neoplasm early and to perform its diagnostic evaluation, and the oncologist collects the material for morphological and immunohistochemical analysis. Patients with this pathology should be monitored by the ophthalmologist throughout the course of treatment to assess treatment effect. Such patients require lifetime monitoring to identify and prevent recurrence. Close cooperation of the ophthalmologist and the oncologist is important in the diagnostic evaluation of orbital tumor disorders and is a prerequisite for treatment success.
References 1.Chissov VI, Dar'yalova SL, editors. [Manual of Oncology]. Moscow: GEOTAR-Media; 2009. Russian. 2.Ahmed S, Shahid RK, Sison CP, Fuchs A, Mehrotra B. Orbital lymphomas: a clinicopathologic study of a rare disease. Am J Med Sci. 2006;331:79–83. 3.Demirci H, Shields CL, Shields JA, Honavar SG, Mercado GJ, Tovilla JC. Orbital tumors in the older adult population. Ophthalmology. 2002;109:243–248. 4.Isaacson PG, Wright DH. Malignant lymphoma of mucosal associated lymphoid tissue: a distinctive type of B cell lymphoma. Cancer. 1983;52:1410–1416. 5.Ishii Y, Tomita N, Takasi H, Ogusa E, Hattori Y, Matsuura S, Matsumoto C, Takemura S, Kuwabara H, Ishigatsubo Y. Clinical features of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue. Hematol Oncol. 2012;30:186–189. 6.Poddubnaya IV, Moskalenko OA, Balakireva YuN. [Non-Hodgkin's lymphomas of the marginal zone]. Sovremennaia onkologiia. 2006;1(8):8-13. Russian. 7.Kozarezova TI, Klimkovich NN. [Pediatric blood disorders: a tutorial]. Minsk: Belorusskaia nauka;2001. Russian. 8.Grishina EE. [Malignant ocular lymphomas. Challenges and Perspectives from the Ophthalmologist’s Viewpoint]. Sovremennaia onkologiia. 2006;8(4):27-29. Russian. 9.Coupland S, Krause L, Delecluse H, et al. Lymphoproliferative disorders of the ocular adnexa. Analysis of 112 cases. Ophthalmology. 1998 Aug;105(8):1430-41. 10.Cahill M, Barnes C, Moriarty P, et al. Ocular adnexal lymphoma– comparison of I MALT lymphoma with other histological types. Br J Ophthalmol. 1999 Jun;83(6):742-7. 11.Nakata M, Matsuno Y, Katsumata N, et al. Histology according to the Revised European–American Lymphoma classification significantly predicts the prognosis of ocular adnexal lymphoma. Leuk Lymphoma. 1999 Feb;32(5-6):533-43. 12.Knowles DM, Jacobiec FA, McNally L, Burke J. Lymphoid hyperplasia and malignant lymphoma occurring in the ocular adnexa (orbit, conjunctiva and eyelids): a prospective multiparametric analysis of 108 cases during 1977 to 1987. Hum Pathol. 1990 Sep;21(9):959-73. 13.Meunier J, Lumbroso–Le Rouich, Dendale R, et al. Conjunctival low–grade non–Hodgkin’s lymphoma. Leuc Lymphoma. 2006 Jul;47(7):1295-305. 14.Khalil HA, de Keiser RJ, Kluin PM, et al. Clinical course and pathologic features of conjunctival non–Hodgkin’s lymphoma. A report of six cases. Graefes Arch Clin Exp Ophthalmol. 1990;228(3):246-5. 15.Cher L. Primary CNS Lymphoma. Cancer Forum. 1998;22(2): 138–40. 16.Connor JM. Problem in Lymphoma Management: Special Sites of Presentation. Oncology (Williston Park). 1998 Feb;12(2):185-91; discussion 192-5. 17.Jensen O.A., Johansen S., Kiss K. Intraocular T–cell lymphoma mimicking a ring melanoma. First manifestation of systemic disease. Report of a case and survey of the literature. Graefes Arch Clin Exp Ophthalmol. 1994 Mar;232(3):148-52. 18.Roos DE, O'Brich PC, Crompton JL. Ocular involvement in primary central nervous system lymphoma: an increasing clinical problem? Australas Radiol. 1993 Nov;37(4):372-4. 19.Medeiros LJ, Harmon DC, Linggood R.M., Harris N.L. Immunohistologic features predict clinical behavior of orbital and conjunctival lymphoid infiltrates. Blood. 1989. 1989 Nov 1;74(6):2121-9. 20.[Non- Hodgkin’s lymphomas (NHL)]. In: Martynov AI, Kokorin VA, Gaewski P, editors. [Evidence-based medicine]. Medycyna Praktyczna: Krakow; 2018. Russian. 21.Armitage JO. Peripheral T-cell lymphoma. In: Canellos GP, Lister TA, Sklar JL, eds. The Lymphomas. Philadelphia: WB Saunders; 1998: 439-48.
Disclosures Conflict of Interest Statement: The authors state that they have no conflict of interest that might bias this work.
|