J.ophthalmol.(Ukraine).2022;5:23-29.
|
http://doi.org/10.31288/oftalmolzh202252329 Received: 08.09.2022; Accepted: 29.09.2022; Published on-line: 27.10.2022 Assessing the early and late impact of excimer laser correction for myopia on the development of dry eye syndrome S. Yu. Mogilevskyy, M. Yu. Zhovtoshtan Shupik National Healthcare University of Ukraine; Kyiv (Ukraine) TO CITE THIS ARTICLE: Mogilevskyy SYu, Zhovtoshtan MYu. Assessing the early and late impact of excimer laser correction for myopia on the development of dry eye syndrome. J.ophthalmol.(Ukraine).2022;5:23-9. http://doi.org/10.31288/oftalmolzh202252329
Background: Today, the global annual volume of excimer laser correction (ELC) is estimated to be 3.6 million procedures. Dry eye syndrome (DES) is a complication of ELC for myopia, and the frequency of DES at 1 month and 6 months after ELC for myopia has been reported to be 60% and 20%, respectively. Purpose: To assess the early and late impact of ELC for myopia on the development of DES. Material and Methods: Sixty-eight myopic patients (136 eyes) were prospectively divided into two groups, group 1 (a Laser-Assisted in Situ Keratomileusis (LASIK) group) and group 2 (a FemtoLASIK group). Patient age ranged from 20 to 44 years. Patients were assessed for DES (ocular surface, tear production, and tear film stability) preoperatively and postoperatively. Patients of group 1 received thin-flap LASIK using the Alcon Wavelight EX500 excimer laser. A 110-µm corneal flap was created by a Carriazo-Pendular microkeratome in group 1 and by an Alcon FS200 femto laser in group 2. Follow-up duration was 12 months. Results: Preoperative function tests showed mild dry eye in some patients of both groups. At 1 month and 3 months after ELC, the frequency of DES increased in group 1 by 75.5% and 63%, respectively, and in group 2, by 76.5% and 64.9%, respectively. At 6 months, the frequency of DES decreased in groups 1 and 2 by 38.7% and 40%, respectively, compared to the 3-month time point. However, 10% of the patients showing no signs of DES preoperatively had persistent DES after ELC. Conclusion: First, the baseline frequency of DES in patients with myopia was 10%. Second, at 1 month and 3 months after ELC for myopia, the frequency of DES increased by 75.5% and 63%, respectively, in the LASIK group, and by 76.5% and 64.9%, respectively, in the FemtoLASIK group. In addition, the frequency did not depend on the laser technique. Third, we noted a gradual decrease in the frequency of DES at late time points after ELC for myopia. At 6 months, the frequency of DES decreased by 38.7% and 40% in groups 1 and 2, respectively, compared to the 3-month time point. Finally, 10% of the patients showing no signs of DES preoperatively had persistent DES after ELC for myopia. Keywords: myopia, excimer laser correction, dry eye syndrome
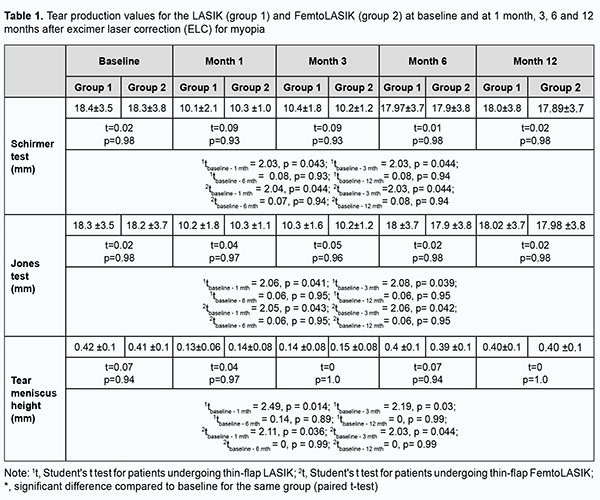
Inroduction Myopia is one of the most common disorders of the eye. More than 2 billion people worldwide have a degree of myopia, 15% of whom have high myopia. In 2020, an estimated 161 million people worldwide were blind or had moderate to severe vision impairment from uncorrected refractive error. By 2050, myopia is expected to affect 5 billion people, more than half of the projected global population [1]. The myopia burden is highest in East Asia and the high-income countries of the Asia-Pacific region (51.6% and 53.4% prevalence in 2020, respectively) but the prevalence is also high in Europe (Western Europe: 36.7%, Central Europe: 34.6%, and Eastern Europe: 32.2%) [2, 3, 4]. Optical methods provide temporary correction for myopia, whereas surgical procedures have been developed for permanent correction of refractive errors. Excimer laser correction (ELC) offers fast recovery of vision with an almost painless postoperative course [5]. Refractive surgery is aimed at safe and predictable improvement to stable target refraction without causing new optical problems. Today, the global annual volume of excimer laser correction (ELC) is estimated to be 3.6 million procedures [6]. ELC is one of the safest surgical procedures [7]. Patient satisfaction rate following laser-assisted in situ keratomileusis (LASIK) has been reported to range from 92–98% [8]. Since Food and Drug Administration (FDA) approval 25 years ago, there has been a progression of technological improvements leading to better outcomes [9]. The ELC procedure is safe and predictable; however, complications may occur during or after the operation. The rate of perioperative complications for LASIK ranges from 0.7% to 6.6%, and these complications are most commonly associated with a microkeratome created flap or femtosecond (FS) laser created flap [10, 11, 12]. Possible postoperative complications include diffuse lamellar keratitis (1-2%); traumatic flap dislocation (1.4%); epithelial ingrowth (< 0.2%); IOP-induced steroid keratopathy (7-10%); undercorrection or overcorrection (3-5%); optical aberrations (e.g., halo) (40%), severe discomfort associated with optical aberrations (< 1%); infectious keratitis (0.03%); macrostriae or microstriae (0.5%); corneal ectasia (0.6%), and dry eye syndrome (DES; 10-20%) [13]. The frequency of DES at 1 month and 6 months after ELC for myopia has been reported to be 60% and 20%, respectively [14, 15]. DES is a multifactorial ocular surface disease associated with etiological factors like tear film abnormalities, tear film hyperosmolarity, ocular surface inflammation and irritation, and neurosensory abnormalities. The global prevalence of DES ranges from 5 to 50% [16]. The estimated numbers of individuals with DED and those with symptoms of DED in Ukraine in 2021 were 2.1 million and 18 million, respectively [17]. A cascade of reactions resulting in tear film instability may be triggered by tear film hyperosmolarity or caused by ocular surface disorders. Several different disorders, including, but not limited to, ocular surface inflammation due to topical preservative toxicity, allergic eye disease, and loss of conjunctival goblet cells or altered mucin expression, due to xerophthalmia, can lead to tear film instability. In addition, it may be induced by ELC [18-20]. We believe that studying post-ELC complications (particularly, DES) is important and may facilitate a reduction in the growing burden of uncorrected myopia through the expansion of the opportunities for adequate and advanced methods of refractive error correction. The purpose of the study was to assess the early and late impact of excimer laser correction for myopia on the development of DES. Material and Methods Approval for the study was obtained from the Bioethics Committee, the Shupik National Healthcare University of Ukraine. The procedures followed were in accordance with the ethical standards of the Helsinki Declaration of the World Medical Association, European Convention on Human Rights and Biomedicine (1977), relevant provisions of WHO’s Constitution, Council for International Organizations of Medical Science, International Code of Medical Ethics (1983), and Ministry of Health Order No. 690, dated 23 September, 2009. This was a prospective, observational, interventional clinical case-control study. Informed consent was obtained from all participants. Sixty-eight myopic patients (136 eyes) were prospectively divided into two groups, group 1 (a LASIK group) and group 2 (a FemtoLASIK group). Patient age ranged from 20 to 44 years, and there were 30 men and 38 women. Of the 136 eyes, 54 (39.7%) were mildly myopic, 50 (36.8%), moderately myopic, and 32 очах (23.5%), highly myopic. In addition, 52 eyes (38.2%) had compound myopic astigmatism of 2 D or less. Patients of group 1 (70 eyes) received thin-flap LASIK using an EX500 Excimer Laser system (Alcon, Fort Worth, Texas), with a 110-µm corneal flap created by a Carriazo-Pendular microkeratome. Patients of group 2 (66 eyes) received thin-flap FemtoLASIK using an EX500 Excimer Laser system (Alcon, Fort Worth, Texas), with a 110-µm corneal flap created by an FS200 femto laser (Alcon). All interventions were performed by one team of surgeons. Preoperative examination included visual acuity, refractometry (particularly, under cycloplegia), keratometry, optical biometry, corneal topography, biomicroscopy, ophthalmoscopy and pupillometry. Postoperative examination included visual acuity, refractometry, keratometry, tonometry, optical and biomicroscopy. Postoperative treatment included topical fluoroquinolon antibiotics and topical dexamethasone for a month. In addition, patients were assessed for DES (ocular surface, tear production, and tear film stability) preoperatively and postoperatively. Tear film stability was assessed by measuring tear break-up time (TBUT). A fluorescein paper strip was moistened with a drop of isotonic saline and placed on the lower lid margin near the external angle of the eye. The tear film was examined using a broad-beam of slit lamp with a blue filter. The interval in seconds elapsing between the last complete blink and the appearance of the first break in the fluorescein stained tear film was determined. The test was performed in triplicate prior to instilling any drops or manipulating the lids, and the average of the triplicate measurements was determined. Fluorescein dye was applied to assess the corneal surface. Fluorescein strips were used as described above. The dye accumulates in the dry defects of the ocular surface. Corneal surface was examined using a broad-beam of slit lamp with a blue filter. Fluorescein staining of the conjunctiva was observed using a broad-beam of slit lamp with a yellow filter. Corneal staining was graded using the Oxford Scheme for grading ocular surface staining in dry eye. Schirmer test, Jones test and strip meniscometry were used to assess the tear production. Jones test was used to assess basal tear secretion. After topical anesthesia, a 35-mm test paper strip was placed at the external margin of the lower lid for 5 minutes, and the length of paper wetting in millimeters was measured. Schirmer test without anesthesia was done to assess reflective tear secretion. A 35-mm test paper strip was placed at the external margin of the lower lid for 5 minutes, and the length of paper wetting in millimeters was measured. Optical coherence tomography (OCT) of the anterior segment was used to measure the inferior tear meniscus height. Statistical analyses were performed using MedStat and MedCalc v.15.1 (MedCalc Software bvba). A paired t-test (two-tailed) was used to analyze the difference between baseline and post-intervention measures. A p-value of < 0.05 was considered statistically significant. Follow-up duration was 12 months. Results At baseline, function tests showed mild dry eye in some patients of group 1 and group 2. Mild abnormalities were shown by the TBUT test in 10% and 12.1% of patients of group 1 and group 2, respectively; Jones test in 10% and 10.6% of patients of group 1 and group 2, respectively; Schirmer test in 8.5% and 10.6% of patients of group 1 and group 2, respectively; meniscometry in 12.8% and 10.6% of patients of group 1 and group 2, respectively. In addition, ocular surface fluorescein staining showed mild irritation in 11.4% and 10.6% of patients of group 1 and group 2, respectively. Therefore, based on the results of the above tests, the baseline frequency of DES was 10% and 10.6% for group 1 and group 2, respectively. At 1 month after ELC for myopia, the frequency of DES increased by 75.5% in group 1 and by 76.5% in group 2, as compared with baseline. These patients complained of pain, foreign body sensation and redness of the eye, tearing, photophobia and sometimes diurnal variations in reduced visual acuity. Of the patients of group 1, 34.2% and 8.5% had mild and moderate tear film instability, respectively, as measured by the TBUT test. In group 2, the frequency of mild tear film instability was 40.9%. Of the patients of group 1, 27.1% and 8.5% had mild and moderate reductions in basal tear secretion, respectively, as measured by the Jones test. In group 2, the frequency of mild reductions in basal tear secretion was 37.8%. Of the patients of group 1, 28.5% and 7.1% had mild and moderate reductions in reflective tear secretion, respectively, as measured by the Schirmer test. In group 2, the frequency of mild reductions in reflective tear secretion was 36.4%. Ocular surface fluorescein staining showed mild irritation in 35.7% and 31.8% of patients of group 1 and group 2, respectively. Moderate irritation as assessed by fluorescein staining score was seen in 5.7% of patients in group 1. Tear meniscus height was low in 35.7% and 34.8% of patients of group 1 and group 2, respectively. At 3 months after ELC for myopia, there was a reduction in the frequency of dry eye symptoms in both groups. The percentage of patients having tear film instability decreased to 31.4%, and no patient had moderate or severe tear film instability, as measured by the TBUT test, in group 1. In addition, the percentage of patients having tear film instability decreased to 30.3%, as measured by the TBUT test, in group 2. There was also a reduction in the frequency of abnormal tear profile in patients in group 2, and the index decreased to 30.3%. Reduced basal and reflective tear secretion (as measured by the Jones test and Schirmer test, respectively) were seen in 25.7% and 27.1% of patients, respectively, in group 1, and 25.8% and 24.2% of patients, respectively, in group 2. Tear meniscus height was low in 28.5% and 27.3% of patients of group 1 and group 2, respectively. In addition, signs of ocular surface irritation were seen in 31.4% and 27.3% of patients of group 1 and group 2, respectively. At 6 months after ELC for myopia, the frequency of dry eye symptoms in both groups was lower compared to previous time points. Shortened tear break-up time was seen in 20% and 19.7% of patients of group 1 and group 2, respectively. Reduced basal and reflective tear secretion were seen in 15.7% of patients in group 1, and 16.6% of patients in group 2. Tear meniscus height was low in 17.1% and 16.6% of patients of group 1 and group 2, respectively. Ocular surface staining was seen in 18.6% and 19.7% of patients of group 1 and group 2, respectively. There was no significant difference in the results of functional tests for dry eye between 12 months after ELC for myopia and previous time points. The frequency of DED did not change significantly over any follow-up time points for either group, and the frequency remained stable over the period from 6 months to 12 months after ELC for myopia. Table 1 shows tear production values for the LASIK (group 1) and FemtoLASIK (group 2) at baseline and at 1 month, 3, 6 and 12 months after ELC for myopia.
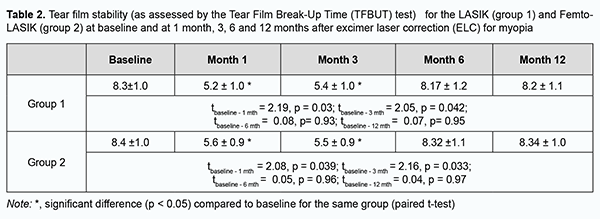
Statistically significant reduction in tear secretion in both groups was seen at 1 month and 3 months (Table 1). At 6 months, the frequency of decreased tear secretion in both groups was almost as high as before operation. There was no significant difference (p < 0.05) in any characteristic between the LASIK and FemtoLASIK groups at any time point. Of note, both ELC techniques (i.e., LASIK and FemtoLASIK) had the same impact on the development of DES. Table 2 shows the tear film instability (as measured by the TBUT test) at baseline and at 1 month, 3, 6 and 12 months after ELC for myopia for both groups. The tear film instability decreased at 1 month and 3 months compared to the preoperative time point (p < 0.05) in both groups (Table 2). At 6 months and 12 months, there was a gradual decrease in the frequency of DES symptoms in both groups.
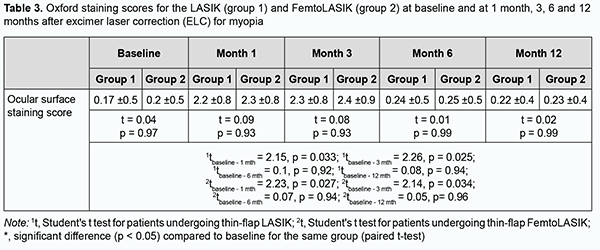
Table 3 presents corneal fluorescein staining scores (Oxford scale, 0-5) at baseline and at 1 month, 3, 6 and 12 months after ELC for myopia, for the LASIK (group 1) and FemtoLASIK (group 2) techniques. The corneal fluorescein staining score increased at 1 month and 3 months compared to the preoperative time point (p < 0.05) in both groups. We noted a gradual decrease in corneal fluorescein staining scores (Oxford scale) in both groups at 6 months and 12 months after ELC for myopia.
Discussion The results of our study demonstrate that ELC for myopia impacts the development of DES. Of note, the impact was mostly temporary and was observed early (at 1 month and 3 months) after intervention. In addition, we found a tendency for gradual regression of manifestations of DES in both groups of the study. The frequency of DES increased fourfold, to 42.7% in group 1, and to 40.9% in group 2, at 1 month after ELC for myopia, and thereafter gradually decreased with time. At 3 months after ELC for myopia, the frequency of DES decreased in groups 1 and 2 by 34.5% and 33.2%, respectively, compared to the previous time point. At 6 months after ELC for myopia, frequency of DES decreased in groups 1 and 2 by 38.7% and 40%, respectively, compared to the 3-month time point and by 61.1% in and 58.2%, respectively, compared to the 1-month time point. There was no significant difference in the frequency of DES between the 6-month and 12-month time points for both groups. However, 10% of the patients of this study showed no signs of DES preoperatively but had persistent DES after ELC. Therefore, further research is required to elucidate the major factor inducing DES. It is generally believed that LASIK induces a greater decrease in tear secretion and corneal sensitivity, with more profound dry eye symptoms than refractive photorefractive keratectomy (PRK) [21]. Complications due to a disruption of corneal sensory innervation are a feature of LASIK and PRK, in part the result of reduced tear secretion, a fall in blink rate, loss of trophic support and changes in tear composition and stability [22, 23]. Punctate keratitis on the flap, but sparing the region of the hinge, has supported a causal role for sensory denervation and neuropathic firing from damaged sensory endings termed LASIK-Induced-Neuro-Epitheliopathy, or LINE [22]. Also, it is suggested, that NGF and other neuropeptides such as substance P or CGRP may be key factors in the syndrome [24]. The two etiologies are not mutually exclusive and it is likely that LASIK DED and LINE can occur together, in which case a clue to the presence of DED is punctate epitheliopathy in that it affects both the LASIK flap and the cornea/conjunctiva outside it, in a distribution typical of DED. Evidence indicated that tear hyperosmolarity could initiate a damaging cascade of inflammation at the ocular surface. Importantly, a given etiology of DES may enter the vicious circle at any point to participate in this process [20]. It is believed that DED may be manifested by the clinical syndrome of pain, foreign body sensation and redness of the eye, reduced visual acuity and punctate keratitis on the flap. This is in agreement with our observations of patients with post-LASIK or post-femtoLASIK DES. It has been reported that persistent post-ELC DES may lead to refractive regression due to epithelial hyperplasia and corneal stroma remodeling [25]. The results of some studies are, however, in disagreement with the theory of neurotrophic iatrogenic impact of LASIK and FemtoLASIK on the development of DES. After PRK, almost 50% of patients reported dry eye and foreign body sensation symptoms, and 20% of patients, transient ocular and eyelid pain, which could be manifestations of subclinical microerosions due to poor early postoperative corneal epithelial cell adhesion. This was confirmed by experimental studies [26]. Corneal epithelial damage is accompanied by a release of cytokines, including interleukin-1 alpha (IL-1α) and FAS ligand, which can induce apoptosis in keratinocytes, support chronic inflammation and aggravate DES [27-29]. Tear hyperosmolarity stimulates a cascade of events in the epithelial cells of the ocular surface, involving MAP kinases and NFkB signaling pathways and the generation of inflammatory cytokines (interleukin-1 [IL-1]α; IL-1 β); tumor necrosis factor- α [TNF-α]) and proteases, such as MMP9. These activate and recruit inflammatory cells to the ocular surface which become an additional source of inflammatory mediators. Such mediators, acting with tear hyperosmolarity itself, lead to a reduced expression of glycocalyx mucins, to apoptotic death of surface epithelial cells and to loss of goblet cells. Goblet cell loss is a feature of every form of DES, reflected by reduced tear mucin levels. Altered expression of glycocalyx mucins, by compromising ocular surface wetting, leads to early tear film breakup. This amplifies or initiates ocular surface hyperosmolarity, which completes the vicious circle and establishes the mechanism that perpetuates the disease [20, 30-38]. The results of the current study are in agreement with the literature data [14, 15]. Studies of tear fluid cytokine and hormone profile may be more valuable in the diagnostic assessment and classification of DES than those determining tear secretion amount and rate. Conclusion First, the baseline frequency of DES in patients with myopia was 10%. Second, at 1 month and 3 months after ELC for myopia, the frequency of DES increased by 75.5% and 63%, respectively, in the LASIK group, and by 76.5% and 64.9%, respectively, in the FemtoLASIK group. Third, we noted a gradual decrease in the frequency of DES at late time points after ELC for myopia. At 6 months after ELC for myopia, frequency of DES decreased by 38.7% and 40% in groups 1 and 2, respectively, compared to the 3-month time point. Fourth, 10% of the patients showing no signs of DES preoperatively had persistent DES after ELC for myopia. Finally, the results of our study demonstrate that LASIK and FemtoLASIK for myopia impacted the development of DES, but the impact was mostly temporary. Further research of changes in biochemical and immunological profile of tear fluid is required to elucidate major factors impacting the development of DES.
References 1.Burton, Matthew J et al. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob Health. 2021 Apr; 9(4):e489-e551. 2.Morgan IG, Ohno-Matsui K, Saw S-M. Myopia. Lancet. 2012; 379: 1739–48. 3.Tideman JWL, Snabel MC, Tedia MS, et al. Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmol. 2016 Dec 1;134(12):1355-63. 4.McCrann S, Loughman J, Butler JS, et al. Smartphone use as a possible risk factor for myopia. Clin Exp Optom. 2021 Jan;104(1):35-41. 5.Barsam A, Allan BDS. Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia. Cochrane Database Syst Rev. 2012 Jan 18;1:CD007679. 6.Jones C. Refractive Surgery Market Report. MarketScope. 2019; 2020:1–283 7.Joffe SN. The 25th Anniversary of Laser Vision Correction in the United States. Clin Ophthalmol. 2021 Mar 17;15:1163-72. 8.Moshirfar M, Shah TJ, Skanchy DF, Linn SH, Kang P, Durrie DS. Comparison and analysis of FDA reported visual outcomes of the three latest platforms for LASIK: wavefront guided Visx iDesign, topography guided WaveLight Allegro Contoura, and topography guided Nidek EC-5000 CATz. Clin Ophthal. 2017;11:135–147. 9.Xia LK, Yu J, Chai GR, Wang D, Li Y. Comparison of the femtosecond laser and mechanical microkeratome for flap cutting in LASIK. Inter J Ophthalmol. 2015;8(4):784–790. doi: 10.3980/j.issn.2222-3959.2015.04.25. 10.Hamill MB et al. 2019-2020 Basic and Clinical Science Course, Section 13: Refractive Surgery. American Academy of Ophthalmology; 2020. pp.130-147. 11.Mogilevskyy SYu, Pavlyuchenko AK. [Causes of failures of excimer laser vision correction]. In: [Proceedings of the Filatov Memorial Lectures conference with international speakers]. May 26-29, 2009. Odesa. Russian. 12.Mogilevskyy SYu, Yakubenko ED, Pavlyuchenko AK. [Tear biochemistry in patients with myopia and myopic astigmatism and its impact on the frequency and nature of post-excimer laser correction complications]. Pytannia eksperymentalnoi ta klinichnoi medytsyny. 2010; 14(2):208-13. Donetsk: DonMU. 13.Linke SJ, Llovet F, Ortega-Usobiaga J, et al. Early (< 3 Months) and Late (> 3 Months) Complications of LASIK. In: Linke SJ, Katz T, eds. Complications in Corneal Laser Surgery. Cham: Springer International Publishing; 2016. 14.Eydelman M, Hilmantel G, Tarver ME, et al. Symptoms and Satisfaction of Patients in the Patient-Reported Outcomes With Laser In Situ Keratomileusis (PROWL) Studies. JAMA Ophthalmol. 2017 Jan 1;135(1):13-22. 15.Gong Q, Li A, Chen L, et al. Evaluation of Dry Eye After Refractive Surgery According to Preoperative Meibomian Gland Status. Front Med (Lausanne). 2022 Apr 25;9:833984. 16.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334-65. 17.Aver’ianova OS, Begimbaieva GIe, Vitovska OP, Golovach IIu, Degterieva OV, Deriapa IV, et al. [Draft decision of expert meeting at the international seminar on dry eye disease in the interdisciplinary context]. Oftalmologiia. Vostochnaia Evropa. 2021;11(2). Russian. 18.Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Biomol Concepts. 2012;3:345-64. 19.Clouzeau C, Godefroy D, Riancho L, et al. Hyperosmolarity potentiates toxic effects of benzalkonium chloride on conjunctival epithelial cells in vitro. Mol Vis. 2012;18:85163. 20.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017 Jul;15(3):438-510. 21.Cohen E, Spierer O. Dry Eye Post-Laser-Assisted In Situ Keratomileusis: Major Review and Latest Updates. J Ophthalmol. 2018 Jan 28;2018:4903831. 22.Medeiros CS, Marino GK, Lassance L, Thangavadivel S, Santhiago MR, Wilson SE. The Impact of Photorefractive Keratectomy and Mitomycin C on Corneal Nerves and Their Regeneration. J Refract Surg. 2018 Dec 1;34(12):790-798. 23.Gjerdrum B, Gundersen KG, Lundmark PO, Potvin R, Aakre BM. Prevalence of Signs and Symptoms of Dry Eye Disease 5 to 15 After Refractive Surgery. Clin Ophthalmol. 2020 Jan 28;14:269-279. 24.Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. 2014 Jan;12(1):32-45. 25.De Paiva CS, Volpe EA, Gandhi NB, Zhang X, Zheng X, et al. Disruption of TGF-b Signaling Improves Ocular Surface Epithelial Disease in Experimental Autoimmune Keratoconjunctivitis Sicca. PLoS ONE. 2011; 6(12): e29017. 26.Mehlan J, Linke SJ, Skevas C, Steinberg J, Giannakakis K, Katz T. Safety and complications after three different surface ablation techniques with mitomycin C: a retrospective analysis of 2757 eyes. Graefes Arch Clin Exp Ophthalmol. 2019 Jan;257(1):217-223. 27.Palme C, Mulrine F, McNeely RN, Steger B, Naroo SA, Moore JE. Assessment of the correlation of the tear breakup time with quality of vision and dry eye symptoms after SMILE surgery. Int Ophthalmol. 2022 Mar;42(3):1013-1020.Crossref PubMed 28.Wilson SL, El Haj AJ, Yang Y. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. J Funct Biomater. 2012 Sep 18;3(3):642-87. 29.Wilson SE. Defective perlecan-associated basement membrane regeneration and altered modulation of transforming growth factor beta in corneal fibrosis. Cell Mol Life Sci. 2022 Feb 21;79(3):144. 30.Wilson SE. Fibrosis Is a Basement Membrane-Related Disease in the Cornea: Injury and Defective Regeneration of Basement Membranes May Underlie Fibrosis in Other Organs. Cells. 2022 Jan 17;11(2):309. 31.Arranz-Valsero I, Soriano-Romanı L, Garcia-Posadas L, Lopez-Gatcia A, Diebold Y. IL-6 as a corneal wound healing mediator in an invitro scratch assay. Exp Eye Res. 2014 Aug;125:183-92. 32.Oliver MM, Fuchs D, Tagscherer KE, et al. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-a-dependent necroptosis driven by RIP1 kinase and NF-kB. Oncogene. 2016 Jun 30; 35(26): 3399–409. 33.Yang Q, Zheng FP, Zhan YS, et al. Tumor necrosis factor-a mediates JNK activation response to intestinal ischemia-reperfusion injury. World J Gastroenterol. 2013 Aug 14;19(30):4925-34. 34.Sullivan BD, Pepose JS, Foulks GN. Progressively Increased Variation in Tear Osmolarity Mirrors Dry Eye Severity. JAMA Ophthalmol. 2015 Dec;133(12):1481-2. 35.Igarashi T, Fujimoto C, Suzuki H, et al. Short-time exposure of hyperosmolarity triggers interleukin-6 expression in corneal epithelial cells. Cornea. 2014 Dec;33(12):1342-7. 36.Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013 Oct;11(4):246-58. 37.Ji YW, Byun YJ, Choi W, et al. Neutralization of ocular surface TNF-a reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye. Invest Ophthalmol Vis Sci. 2013;54:7557–66. 38.Sakimoto T, Sugaya S, Ishimori A, Sawa M. Anti-inflammatory effect of IL-6 receptor blockade in corneal alkali burn. Exp Eye Res. 2012 Apr;97(1):98-104. Disclosures Corresponding Author: M. Yu. Zhovtoshtan, Email: mzhovtoshtan@gmail.com Author Contribution: Mogilevskyy S.Yu.: Conceptualization; Writing – review & editing; Zhovtoshtan M.Yu.: Methodology; Writing – review & editing. All authors analyzed the results and the final version of the manuscript was approved by all authors prior to submission. Funding sources: No external funding sources were used for this study Conflict of interest: The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Study Participants: Informed consent was obtained from all participants. Approval for the study was obtained from the Bioethics Committee, the Shupik National Healthcare University of Ukraine. The procedures followed were in accordance with the ethical standards of the Helsinki Declaration of the World Medical Association, European Convention on Human Rights and Biomedicine (1977), relevant provisions of WHO’s Constitution, Council for International Organizations of Medical Science, International Code of Medical Ethics (1983), and Ministry of Health Order No. 690, dated 23 September, 2009.
Abbreviations: DES, dry eye syndrome; ELC, excimer laser correction; FDA, Food and Drug Administration; FS, femtosecond laser; LASIK, Laser-Assisted in Situ Keratomileusis; PRK, Photorefractive Keratectomy
|