J.ophthalmol.(Ukraine).2022;5:42-46.
|
http://doi.org/10.31288/oftalmolzh202254246 Received: 11.09.2022; Accepted: 11.10.2022; Published on-line: 27.10.2022 Diagnostic assessment by fluorescein visualization of skin cancer lesion margins and surgical treatment of malignant skin tumors of the external nose A. F. Yevcheva Odesa National Medical University Odesa (Ukraine) TO CITE THIS ARTICLE: Yevcheva AF. Diagnostic assessment by fluorescein visualization of skin cancer lesion margins and surgical treatment of malignant skin tumors of the external nose. J.ophthalmol.(Ukraine).2022;5: 42-46. http://doi.org/10.31288/oftalmolzh202254246 Purpose: To assess the diagnostic information value of chlorin e6 fluorescence visualization of cancer lesion margins in the radical surgical removal of malignant lesions of the external nose. Material and Methods: Thirty patients were examined and underwent surgery for malignant cancer lesions of the external nose at the department for ear, nose and throat cancer during 2017 to 2020. Half of these patients (group 2) received and the rest (group 1) did not receive a preoperative injection of Klein solution and fluorescence visualization of cancer lesion margins. Results: Cancer lesion margins were visualized by fluorescence with chlorin e6 via deep green staining of tumor tissue. The tumor focus appeared violet, and the tumor periphery, crimson when chlorine-e6 fluorescence was excited with 630-633 nm light from a HeNe laser. Because there was no change in tumor lesion margins when compared with visualization without exposing the lesion to laser radiation, exposing the lesion to laser radiation is not mandatory. At three years after surgery, the non-recurrence rate was for 100% for the group of patients who underwent surgery with preoperative injection of Klein solution and fluorescence visualization of cancer lesion margins, and 73.4% for the group of patients who underwent surgery without chlorin e6 fluorescein visualization. Two years after primary surgery, 4 (26.6%) of group 1 patients were diagnosed with recurrent superficial cancer of the skin at the cartilage-bone interface. Conclusion: First, the recurrence rate was 26.6% (4 of 15 patients) for the group of patients who underwent surgery without chlorin e6 fluorescein visualization, which was significantly higher than for the group of patients who underwent surgery with chlorin e6 fluorescein visualization (0 of 15 patients, z = 2.091; р = 0.037). Second, the use of chlorin e6 fluorescein visualization of the margins of a skin lesion of the external nose in patients with malignant tumors of the external nose with tumor spread to the tissues of the medial angle of the eye and eyelid enabled a radical approach to surgical treatment to be employed in 100% of cases. Keywords: malignant tumors, external nose, chlorin e6, fluorescein visualization
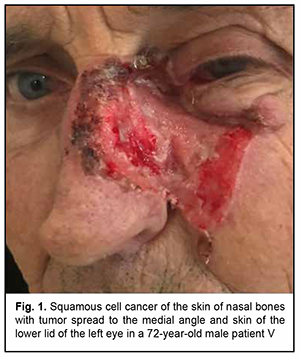
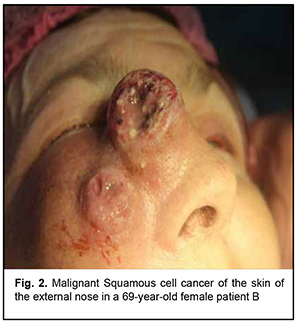
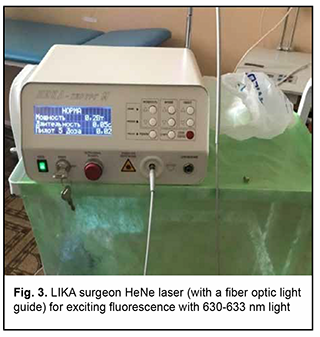
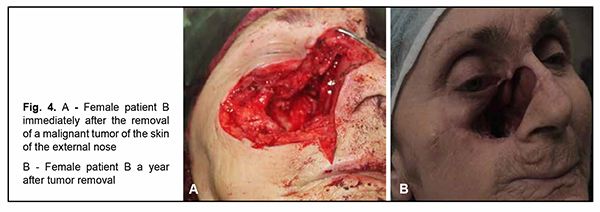
Introduction Malignancies are an important medical, biological, social and economic problem globally and particularly in Ukraine. In 2012, superficial head and neck cancer was the most common type of oncological disease in Ukraine, accounting for 10.3% of all oncological cases. The estimate of annual incidence of superficial head and neck cancer in Ukraine is 44.5 cases per 100,000 population [1, 2, 3]. In recent years, there has been an increase in the global incidence of superficial epithelial malignancies, particularly, basal cell carcinoma and squamous cell skin carcinoma, the two most common carcinomas of the head [4, 5], with the incidence of newly diagnosed cases per year of about 100 per 100,000 population [1, 2, 3, 6, 7, 8, 9]. Because topical and differential diagnosis of chronic (pre-cancer) conditions (actinic keratosis, Bowen’s disease and cancer in situ) is rather difficult, the diagnosis of nasal skin cancer is a challenge, although clinical symptoms in the form of changes in the color and size of the neoplasm can be early observed [4, 10, 11, 12]. The diagnostic assessment of superficial head tumors is important, because late diagnosis of nasal skin cancer results in tumor spread to the skin of the medial angle of the eye and lower eyelid, making the surgical treatment more difficult and worsening the quality of life. Unfortunately, patients do not seek care early in their illness, although early clinical signs of the tumor can give evidence of the aggressiveness of the process [1, 2, 5, 9, 13, 14, 15, 16]. Consequently, in the presence of a superficial skin tumor of the external nose with signs of malignancy, the morphological type of the lesion should be promptly identified, and the lesion should be treated with a radical approach to prevent tumor spread to the ocular tissue and face. There are two morphology-based methods of malignant skin tumor removal: the first is image-guided skin tumor removal taking in account the coefficient of radicality, and the second is Mohs micrographic surgery, a technique for excision and analysis of skin cancers employed by specially trained dermatologists. The Mohs procedure involves surgically removing skin cancer layer by layer and examining the tissue under a microscope until healthy, cancer-free tissue around the tumor is reached. The main advantage of Mohs surgery is that it offers precise microscopic control of the entire tumor margin while maximizing conservation of healthy tissue. In our opinion, Mohs micrographic surgery is more appropriate for the removal of tumors of the trunk, in which there is a possibility for tissue mobilization with a retreat from the tumor taking in account the coefficient of radicality, and using surrounding tissues for defect closure. In addition, Mohs micrographic surgery requires a specially trained dermatologist and more time for performing additional morphological studies to avoid surgical failure in the form of facial deformities. Taken together, the above confirms the importance of developing an advanced method for preoperative objective visualization of skin cancer lesion margins in radical tumor removal surgery [2, 3, 4, 10]. Fluorescence imaging is currently the most effective high-quality technique for visualization of cancer lesion margins and widely used by dermatologists and oncosurgeons in photodynamic therapy and tumor removal surgery. Chlorin E6 is a photosensitizer authorized in Ukraine, and we used chlorin E6 fluorescence imaging in the surgical removal of cancer lesions of the external nose and ear. The purpose of the study was to assess the diagnostic information value of chlorin E6 fluorescence visualization of cancer lesion margins in the radical removal of malignant lesions of the external nose. Material and Methods Thirty patients (13 women and 17 men) with skin cancer tumors of the external nose were examined, treated and followed at the department for ear, nose and throat cancer of Odesa municipal clinical hospital No. 11 during 2017 to 2020 and were involved in the study. Their age ranged from 55 to 75 years. Patients were divided into two groups of 15 patients each depending on the type of tumor growth and the method used for objective visualization of the cancer lesion margins in the removal of lesions of the external nose. Group 1 was composed of 15 patients with exophytic skin lesions of the external nose that underwent tumor removal within healthy tissue margins without fluorescence visualization. Tumor lesion size ranged from 1-3 to 8-10 cm (Figs 1, 2). Group 2 was composed of 15 patients with endophytic and infiltrating skin lesions of the external nose with lesion size ranging from 2-3 to 8 cm. Patients underwent a comprehensive clinical study (dermatoscopy, morphological examination, differential thermal analysis, and clinical tests), and duration of skin lesions and changes in lesion color, size and/or shape were assessed. In addition, they were consulted by a dermatologist. For control purposes, surgical tumor removal was accompanied by fast histological assessment of resection margins. The duration of surgery was 60 minutes, and the duration of fast histological assessment, 20 minutes. In group 1, the tumor with 1.2-1.5 cm- healthy margins was surgically excised, with fast histological assessment showing the absence of malignant cells in resection margins. Resection depth was determined visually. In group 2, ce6 was used for fluorescence imaging of tumor lesion margin, with ce6 randomly accumulated in tumor tissue. The photosensitizer was injected into tumor lesions at a dose of 1.0-3.0 μmg/kg within 3-5 minutes, and the result was assessed in 50 to 60 minutes. Five minutes prior to surgery, Klein solution (a mixture of 1% Lidocaine and 2-3 drops of Adrenaline) was injected under the base of the tumor to prevent intraoperative tumor metastasis and hemostasis. The amount of Klein solution injected depended on the area of tumor lesion, with an average amount ranging between 5.0 to 8.0 ml. This injection resulted in spasm of blood and lymph vessels, leading to hemostasis. Thereafter, the tumor lesion was excised within the margins of staining, taking into account the coefficient of radicality, and fast histological assessment of resection margins was performed. The depth of excision was determined by the area of deep green staining. The fluorescence was excited with 630-633 nm light from a HeNe laser (Fig. 3), and the fiber optic spectrofluorometer LESA-01-BIOSPEC with the fiber optic light guide was used for fluorescent visualization of the tumor lesion. During the examination, the fiber optic light guide was directed at a right angle to the tumor surface. The spectra seen in the center and periphery of the lesion and those seen in visually healthy tissues were analyzed for the form and amplitude of the signal. Fluorescence intensity area (S2), area of laser radiation reflected from tissues (S1), and the ratio of these two characteristics (S2/S1, the coefficient of radicality) were determined. Fluorescence of different skin regions (e.g., center and periphery of the lesion and healthy skin regions) was assessed based on the S2/S1 ratio. This allows determining the amount of photosensitizer accumulated not only in the tumor proper, but also in cases of malignant process. In addition, the total lesion area was assessed. An LED radiation system (wavelength, 665 nm, radiation power, 40 mV/cm2) with a video recording system was used for this purpose. Statistical analysis was conducted using Primer Biostatistics software. Z-ratios for proportions were used for comparison. Results Based on histological examination of tumor biopsy specimens, among group 1 patients, 6 (40%) had a T2N0M0 and G1 grade tumor, 7 (46.6%), a T3-4N0M0 and G2 grade tumor, and 2 (13.3%), a T4N0M0 and G3 grade tumor with an alar cartilage defect. In addition, there was a malignant tumor spread to the skin of the medial angle of the eye and lower eyelid in 4 patients. In group 2 patients, sparing biopsy was performed in nodal elements preoperatively. Among group 2 patients, 8 (53.3%) had a T2N0M0 and G1 grade tumor, 6 (40%), a T3-4N0M0 and G2 grade tumor, and 1 (6.7%), a T4N0M0 and G3 grade tumor with a large alar cartilage defect and a malignant tumor spread to the skin of the medial angle of the lower eyelid (Figs. 1, 2, 4).
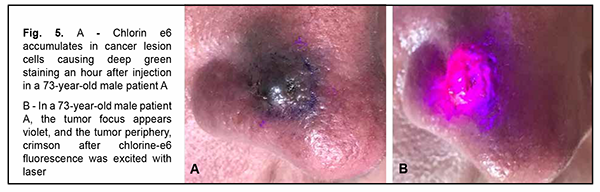
Deep green staining of the tumor was seen, clearly showing tumor lesion margins, 50 to 60 minutes after injection of chlorin e6 in tumor tissue (Fig. 5A).
The tumor focus appeared violet, and the tumor periphery, crimson when the tumor lesion was exposed to laser radiation (Fig. 5B). There was no change in tumor lesion margins when compared with visualization without exposing the lesion to laser radiation. Because laser radiation just confirmed the lesion margins, exposing the lesion to laser radiation is not mandatory. Thereafter, a diamond blue marker was applied to the lesion margins. With this done, the tumor was excised along the external diamond blue margin with the surrounding stained tissue. In both groups, with the results of the histology of the resection margins being negative for malignancy, the defect, if any, was covered with a flap. In addition, in both groups, the postoperative course was unremarkable, and sutures were removed on day 8. In 3 (20%) of group 1 patients with exophytic skin lesions of the external nose, the intervention was completed with a complete lesion removal due to tumor spread to the triangular cartilage and nasal bone. Patients were satisfied with their quality of life and refused a reconstructive defect closure. Two years after primary surgery, 4 (26.6%) of group 1 patients were diagnosed with recurrent superficial cancer of the skin at the cartilage-bone interface with evidence of as large as 1.3-cm star-shaped lesion. In these patients, the defect was closed with local tissue flaps, and postoperative period was unremarkable. Therefore, in group 1, the treatment non-recurrence rate was 73.4%. Group 1 patients showed no signs of recurrence or metastasis over 3 years after surgery, and had a 3 year survival rate of 100%. In group 2 patients, chlorin e6 was injected for fluorescein visualization of skin cancer lesion margins, and Klein solution was injected under the base of the tumor to prevent intraoperative tumor metastasis and hemostasis. These patients showed no signs of recurrence over 3 years after surgery, with a treatment efficacy rate of 100%. There was a significant reduction in differences in late recurrence rate in group 2 compared to group 1 (Z = 2.091, р= 0.037). Discussion The course of malignant superficial skin tumors in the external nose depends on tumor growth type. In patients with endophytic and infiltrating skin lesions of the external nose, the course is complicated by tumor spread to the nasal cartilage and bone tissues, the tissues surrounding the eye, the cranial cavity and brain, and metastasis, most commonly, to the lungs [17]. In these cases, extended surgical interventions have to be performed, and may lead to postoperative defects, and, consequently, reconstructive plastic procedures. When performing radical surgical intervention, it is important to determine skin cancer lesion margins not only visually, but also by objective assessment methods. One of these methods is fluorescein visualization of the margins of a skin lesion of the external nose using chlorin e6, the method we have used for the first time intraoperatively [10]. There have been reports on color visualization of skin tumor lesions via an intravenous approach and in photodynamic therapy [5, 6, 9, 11], but, to the best of our knowledge, there have been no reports on color visualization of skin tumor lesions in the surgical treatment of malignant skin cancer lesions of the external nose. Our surgical treatment for malignant skin lesions of the external nose with tumor spread to the medial angle of the eye and eyelid with chlorin e6 fluorescein visualization in 15 patients allowed to achieve 100% radicality and efficacy (with no signs of tumor extension or recurrence) over the 3-year follow-up period. The results obtained may contribute to improvements in the quality of intraoperative diagnostic assessment of skin lesion margins, surgical treatment efficacy and quality of life of patients. Conclusion First, the recurrence rate was 26.6% (4 of 15 patients) for the group of patients who underwent surgery without chlorin e6 fluorescein visualization, which was significantly higher than for the group of patients who underwent surgery with chlorin e6 fluorescein visualization (0 of 15 patients, z = 2.091; р = 0.037). Second, the use of chlorin e6 fluorescein visualization of the margins of a skin lesion of the external nose in patients with malignant tumors of the external nose with tumor spread to the tissues of the medial angle of the eye and eyelid enabled a radical approach to surgical treatment to be employed in 100% of cases.
References 1.Anishchenko IS, Vazhenin AV. [Squamous skin cancer: clinical features, diagnostic assessment and treatment], Cheliabinsk: Ural Publishing House; 2000. Russian. 2.Buiko AS, Safronenkova IA, Piterova OV. [T3 and T4 tumors of lid-skin epithelium: whether to use combined treatment or excision alone]. Oftalmol Zh. 2002; 1: 30-34. Russian. 3.Buiko AS, Elagina VA, Safronenkova IA. [Combined treatment of T3 and T4 malignant tumors of lid-skin epithelium]. In: [Proceedings of the international conference on Tumors and Tumor-like Conditions of the Eye]. October 20-22. 1998, Moscow. Russian. 4.Kolesnik OO, editor. Cancer in Ukraine 2015–2016: Bulletin of the National Cancer Register of Ukraine. Kyiv: National Cancer Institute; 2017. 5.Lukach EV, Chepurna OM, Pashkovsky VM. [Treatment of basal cell carcinoma of the external nose by photodynamic therapy]. Rynologiia. 2017 (1): 65-7. Ukrainian. 6.Kochneva EV. [Results of phase II trial of treatment with the photosensitizer radakhlorin for basal cell skin cancer in Cheliabinsk Municipal Hospital No.1]. Rossiiskii bioterapevticheskii zhurnal. 2005; 4 (4): 92–95. Russian. 7.Lamotkin IA. [Atlas of Clinical Dermatooncology]. Moscow: BINOM; 2011. Russian. 8.Chaqas FSC, Silva B de S. Mohs micrographic surgery: a study of 83 cases. An Bras Dermatol. 2012 Mar-Apr;87(2):228-34. 9.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992 Jan; 55(1):145–57. 10.Ievcheva AF. [Current diagnostic assessment of malignant tumors of the external nose and ear]. Otorinolaringologiia. 2019; (2-3); 47–52. Ukrainian. 11.Kapinus VN, Kaplan MA, Spichenkova IS, et al. [Photodynamic therapy with chlorine e6 for squamous cell skin cancer]. Lazernaia meditsina. 2012;16:2:31-34. Russian. 12.Kuhne K, Konz V. [Current recommendations for therapy for basal cell and squamous cell skin cancer]. Dermatolog. 2012; 3:179-86. Russian. 13.Vit VV. [Tumors of the eye]. Vol. 1. Odesa: Astroprint; 2009. Russian. 14.Paches AI. [Tumors of the head and neck]. Moscow: Meditsina;2000. Russian. 15.[Uniform clinical protocol of primary, secondary (specialized) and tertiary (highly specialized) care for basal cell skin cancer. Order of the Ministry of Health of Ukraine No. 266 dated 28.03.2016]. Ukrainian. http://mtd. dec. gov. ua/images/dodatki/2016 – 246- BRshkiry 2016 246 YKPMD- BKR. pdf. 16.Gantsev ShKh, Iusupov AS. Squamous cell skin cancer. Prakticheskaia onkologiia. 2012; 13(2): 87-9. Russian. 17.Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003 Aug; 44(3): 159-166.
Disclosures Corresponding Author: Angelina Yevcheva, email: esebuat11@gmail.com Author Contribution: The author acknowledges sole responsibility for the following: conception and design of the study, acquisition of data, analysis and interpretation of results, and drafting of the manuscript. Funding Sources: There are no external sources of funding. Conflict of Interest: The author declares that she has no conflicts of interest that could influence her opinion on the subject matter or materials described and discussed in this manuscript.
|