J.ophthalmol.(Ukraine).2022;6:50-58.
|
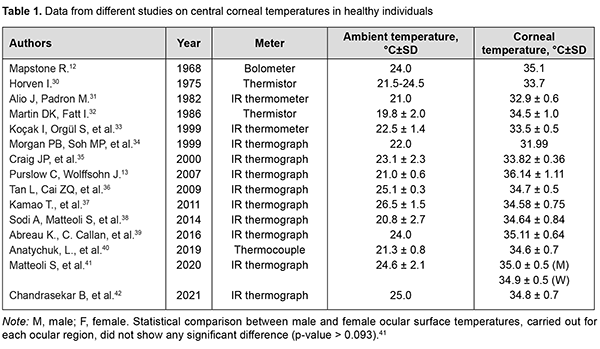
http://doi.org/10.31288/oftalmolzh202265058 Received: 23.10.2022; Accepted: 29.11.2022; Published on-line: 21.12.2022 Heat exchange in the human eye: a review O. S. Zadorozhnyy 1, A. R. Korol 1, V. O. Naumenko 1, N. V. Pasyechnikova 1, L. L. Butenko 2 1 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine" 2 Odesa National Medical Institute Odesa (Ukraine) TO CITE THIS ARTICLE: Zadorozhnyy OS, Korol AR, Naumenko VO, Pasyechnikova NV, Butenko LL. Heat exchange in the human eye: a review. J.ophthalmol.(Ukraine).2022;6:50-58. http://doi.org/10.31288/oftalmolzh202265058 Thermal homeostasis is required in order to ensure that the normal function of the human body is maintained under various environmental conditions. Various pathological processes impacting metabolism in tissues and organs (e.g., the human eye) are accompanied by changes in relative internal heat balance. Although numerous relevant studies have been conducted, heat exchange processes in the human eye have not been yet sufficiently investigated. Further research on the features of heat exchange in the eye is required not only to improve our knowledge in the field of physiology of the eye, but also to use the data obtained for developing novel advanced techniques for eye disease diagnosis and treatment. Keywords: heat exchange in the eye, ocular surface temperature, heat flux, intraocular temperature, mathematical modeling The human organism is capable of maintaining homeostasis, a relative stability of its internal environment, while adapting to continuously changing external conditions, with the physiological parameters of tissue metabolism being carefully controlled within very narrow ranges. Temperature is a fundamental parameter of metabolism.[1, 2]. A relative stability of the internal thermal environment of the body is required for normal metabolism and enables normal functioning of all organs and systems.[1, 3, 4]. There is an ongoing process of energy exchange in the form of heat to support homeostasis in the human. Heat exchange processes enable a balance of the amount of heat produced and the amount of heat released. Heat energy production in the body goes on continuously in the course of exothermic metabolic reactions, with an amount of heat production depending on the activity of metabolism.[5]. Excessive heat is released into the surrounding environment by infrared (IR) electromagnetic radiation, thermal conduction, evaporation and convection. [6, 7]. Heat exchange in biological systems may be considered within the framework of laws of thermodynamics which describe in general terms how energy is transformed and transferred. Biological systems are open thermodynamic systems which strive to become thermally balanced and in which heat transfer occurs continuously.[8-10]. Assessment of heat exchange processes is based on temperature and heat flux measurements. Temperature is a scalar physical quantity which characterizes a condition of heat balance for a thermodynamic system. Heat flux or heat transfer requires a temperature gradient. The heat flux, contrary to temperature, has a certain direction and is always directed toward lower temperature.[8]. The heat flux characterizes heat exchange power, i.e., the flow of energy transferred through the surface of the body per time unit.[11]. The major source of heat for the eye is from the flow of blood circulating in the choroid. Blood enters the eye at a temperature that equals the intracranial temperature, causing a temperature gradient. This induces a heat flux directed toward lower temperature, i.e., toward the cornea that contacts with the external environment. Heat transfer in the eye occurs mainly by thermal conduction and by convection from the aqueous to the surrounding tissues in the anterior and posterior chambers.[7]. The heat distributed across ocular tissues is lost to the environment through the cornea and conjunctiva mostly by radiation, convection and evaporation. The more intensive the circulation, the larger amount of heat is transferred to ocular tissues. The flow of blood circulating in the iris and ciliary body is another source of heat for the eye but is smaller than that circulating in the choroid.[12-14]. Body temperature measurement is a practice that has been used for centuries by men of medicine. Hippocrates provided descriptions of certain diseases also by differentiating between hot and cold objects as early as 400 BC. Not until the invention of the thermometer by Galileo was it possible to make quantitative temperature measurements.[3]. Interest in eye temperature spans over decades, since the time of Dohnberg, who attempted to measure the ocular surface temperature (OST) with a mercury thermometer, as far back as 1876.[15]. Zeiss was the first to report on heat IR radiation measurements on the corneal surface.[16]. Later Mapstone continued research in this field.[17]. The OST can be measured by contact thermometry through temperature sensors (thermistors and thermocouples) requiring a direct contact with the cornea or conjunctiva. Liquid crystal thermography is a contact method for the measurement of OST. [18, 19]. Temperature sensors may be used as components of a smart contact lens.[20, 21]. The OST can be measured without direct contact, by measurement of IR radiation from the ocular surface. IR thermometry or thermography methods are used for this purpose. [22, 23]. Recently, there have been advances in the development of means for measuring heat flux and heat flux density, e.g., in ophthalmology.[24]. The intraocular temperature can be measured only invasively, e.g., by introducing a measuring probe into the anterior chamber of vitreous cavity of the experimental animal.[25, 26]. It is feasible to measure the intraocular temperature in the clinical setting only under conditions of surgery in the presence of already formed surgical approach, e.g., through a vitrectomy port. [27, 28]. There are reports on implantation of intraocular sensors during cataract surgery in eyes with primary open-angle glaucoma (POAG), which allow long-term monitoring of intraocular temperature (IOT) in the human eye [29]. Temperature of and heat flux from the external ocular surface The surface temperature of the central cornea in healthy individuals has been reported to range widely, from 32°С to 36°С (Table 1). There is a progressive increase in temperature from the corneal centre to periphery [13, 38, 42-44], with the limbus being warmer than corneal centre by 0.45–1.0ºC [45] due to the absence of corneal vascularization and continuous tear film evaporation at the surface.
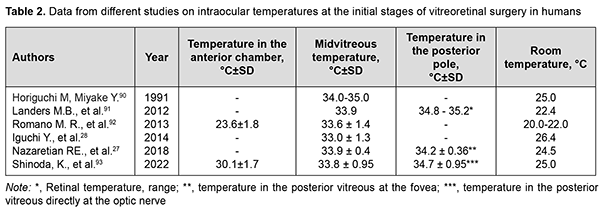
OST depends on the ambient environmental conditions like air velocity and temperature. A linear relationship was found between corneal surface temperature in humans or animals and ambient environment temperature in the absence of air motion.[2, 12]. Room temperature was shown to influence OST; a rise in 1 °C room temperature may lead to an increase of 0.15 °C to 0.2 °C in OST.[46]. OST decreases with a decrease in ambient temperature and an increase in air velocity.[2, 47] A change in relative air humidity has little effect on the corneal surface temperature.[48]. OST also is affected by the state of the tear film.[39, 45, 46, 49-52]. Corneal surface temperatures are lower [39,49,52], whereas ocular surface cooling rates are higher [39,49,52] in patients with dry eye than in healthy individuals. The prevailing factors underlying the pathogenesis of dry eye syndrome can affect OST and changes in OST with time.[39, 49, 52]. Eyelids undoubtedly can have an impact on OST, and blinking causes dynamic changes in OST.[12, 13]. Blinking restores the tear film and facilitates the contact of the ocular surface with the highly vascularized palpebral conjunctiva. Early after a blink, the OST is close to the body temperature.[13]. In a study by García-Porta and colleagues [53], central corneal temperature decreased immediately after opening the eye, and the eyes cooled with an average decrease of 0.24°C during the first second in healthy subjects. A slower decrease was then observed between 1 and 8 seconds. OST increases when contact lenses are worn. OST beneath a contact lens immediately following contact lens wear was significantly greater compared to non-lens wearers, with lens material having the greatest effect on an increase in the corneal surface temperature.[32, 45]. Dilation of eye showed increase in OST for both normal subjects and diabetic retinopathy patients. Dilation studies showed an average increase of 0.82 ± 0.13 °C in cornea.[42]. There was also an increase in density of the heat flux from the ocular surface with eye dilation.[24]. It is likely that the iris, due to its weak blood flow and the lower temperature of the aqueous of the anterior chamber, partially blocks the outflow of heat from the choroid. [14]. No significant interocular difference in OST has been reported in animals and humans.[30, 33, 41, 42, 54, 55]. Morgan and colleagues [34] found that 95 percent of the normal population have an interocular temperature difference of 0.60 degrees or less. A positive correlation between body temperature and OST has been reported in literature. Previous studies have shown that a 1°C increase in body temperature results in a 0.98 °C increase in OST.[49]. It is known that corneal surface temperature is lower than internal body temperature.[45, 54] Koçak and colleagues [33] reported that average corneal temperature varied significantly during the day (p < 0.0001), with lower corneal temperature readings during the morning hours compared to those obtained during the afternoon. There is circadian rhythmicity of human body temperature with a range of 0.8-1°C between night (minimum) and day (maximum) temperatures.[56, 57]. OST decreases with age [31], with a reduction of 0.01°C–0.023°C per year and it is more evident among middle-aged subjects and above.[58]. An average decrease in temperature at the center of the cornea between healthy young individuals of 21-30 years and individuals of 51-60 years was of approximately 1°С. [42]. In healthy individuals, the density of heat flux from the eye also decreases with age.[40]. This is likely to be associated with age-related atrophic changes in the choroid and reduced choroidal blood flow.[59]. In healthy people, the density of the heat flux of the eye on the surface of the cornea is a function of the choroidal blood flow and thickness.[40, 60]. The OST in patients with age-related macular degeneration (AMD) was found to be lower than in healthy individuals of the same age.[38] This is likely to be associated with reduced choroidal thickness in patients with AMD.[61]. In patients with diabetic retinopathy (DR), Chandrasekar and colleagues found a lower OST compared to healthy individuals. [42]. In our previous study, a lower density of heat flux in eyes with the proliferative DR compared to the non-proliferative DR was detected. [62]. This may be explained by severely abnormal ocular hemodynamics in proliferative DR. The results obtained were in agreement with those of others (particularly, the choroidal thickness in proliferative DR was lower than in non-proliferative DR). [63]. At the same time, we did not observe that OST differs significantly in patients with proliferative and nonproliferative DR [62], which indicates the advisability of measuring the density of heat flux along with OST for a comprehensive assessment of the heat transfer of the eye. There have been reports on the effects of retrobulbar hemodynamics on OST.[64, 65]. In a study of ocular temperature in carotid artery stenosis by Morgan and colleagues [66], corneal surface temperature was cooler at the side of the stenosis. Sodi and colleagues [67] found that, in patients with central retinal vein occlusion (CRVO), ischemic CRVO eyes showed lower temperatures than nonischemic ones. Therefore, insufficient delivery of blood to the eye can lead to a low OST. Inflammation is known to affect blood circulation and metabolism, leading to abnormal thermoregulation.[68] In a study by Efron and colleagues [69], conjunctival hyperemia was significantly correlated with conjunctival temperature. Inflammatory eye disease is commonly characterized by an increased ocular surface temperature. [34, 70-72]. OST values were significantly lower in patients with primary open-angle glaucoma (POAG) than in healthy controls.[65]. In a study by García-Porta and colleagues [53], on opening of the eye, subjects with glaucoma showed significantly cooler temperatures in the central cornea compared to healthy controls. In subjects with glaucoma, the eyes cooled significantly faster. Those authors believe that their results support the hypothesis that both the stability of the tear film and changes in the ocular blood supply in subjects with glaucoma play an important role in thermal dynamics of the ocular surface. On the other hand, Leshno and colleagues [73] found that eyes with glaucoma had a significantly higher OST compared to controls. The authors [73] concluded that differences in the OST between glaucomatous and normal eyes strengthen current thinking that inflammation affects the pathophysiology of glaucoma. OST and heat flux density values were lower for eyes with neovascular glaucoma in the presence of DR than for non-glaucomatous fellow eyes. It may be hypothesized that low ocular heat exchange characteristics are caused by limited ocular hemodynamics and hampered metabolism due to high IOP. In addition, in all cases, active inflammation had no decisive effect on heat radiation from the eye.[74, 75]. There was an increase in heat radiation from the corneal surface after IOP decrease in these patients.[75] .An increase of IOP was found to be related to a contemporary decrease of ocular perfusion pressure and OST in monkeys.[76]. Some IOP-reducing medications may lead to a reduction in OST in patients with glaucoma.[77]. This is likely caused by the influence of these drugs on the blood flow to the anterior segment of the patient's eye. Thus, it has been reported previously on thermography evidence of the influence of vasoconstrictors and vasodilators on the OST.[78]. An increased OST is not uncommon after eye surgery. An increase in corneal surface temperature has been reported after surgery for glaucoma [79] and cataract [80], and after vitreoretinal surgery. [81]. This is likely to be caused by postoperative inflammatory response in ocular tissues. OST monitoring can be successfully employed during epibulbar tumor cryosurgery. It has been reported on the use of real-time IR thermography for determining the duration of cryogenic exposure not leading to excessive cooling of the surrounding ocular structures, particularly, the ciliary body and cornea.[82]. Corneal surface temperature changes can be monitored for assessing an increase in corneal temperature during excimer-laser refractive surgery.[83-85]. IR thermography may be useful for monitoring patients after corneal transplant surgery for early detection of transplant failure [86], and for control of filtering bleb formation after glaucoma surgery. [23]. Intraocular temperature (IOT) There has been a long history of attempts to assess IOT in experimental animals. In a rabbit study by Schwartz and Feller [26], average difference between temperature at the retina and at the outer surface of the cornea was 5.01 ºC. They found no significant interocular difference in IOT in animals.[26]. May and colleagues [87] confirmed the existence of an IOT gradient in the rabbit. There have been reports on the effects of the ambient temperature on the IOT in experimental animals.[88, 89]. Data from different studies on intraocular temperatures at the initial stages of eye surgery in humans are presented in Table 2. These studies confirmed the existence of an IOT gradient in humans.
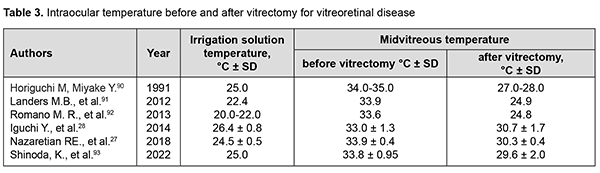
Thus, the temperature in the anterior chamber is lower than that of the vitreous cavity, likely due to the following factors: some heat release to the ambient; partial absorption, by the iris, of the heat produced mostly in the posterior segment of the eye; the lower heat conduction of the lens compared to the surrounding tissues prevents significant heat transfer from the posterior segment to the anterior segment of the eye.[14, 94]. In a study by Mansouri and colleagues [29], patients underwent implantation of an intraocular sensor during cataract surgery,which made it possible to assess long-term IOT variations in eyes with POAG. The results showed significant short-term and longterm fluctuations of IOT. Particularly, on average, IOT was significantly higher on Sundays than on any other day of the week, and over the year, IOT followed a clear seasonal pattern. Significant IOT fluctuations take place the course of vitreoretinal surgery. The temperature of irrigation solutions used in standard vitrectomy is substantially lower than that of the intraocular media, and commonly corresponds to the ambient operating room temperature. Consequently, irrigation in vitrectomy is performed under conditions of uncontrolled IOT and retinal temperature reduction.[27]. Data from different studies on IOT in the course of vitreoretinal surgery are presented in Table 3.
Therefore, vitrectomy is performed under conditions of uncontrolled local ocular hypothermia, with the severity of hypothermia depending on irrigation solution temperature. A fast uncontrolled increase in intravitreal temperature takes place after discontinuation of irrigation in vitrectomy.[81, 95]. Effects of these temperature fluctuations and concomitant vascular responses in ocular tissues have not been completely investigated and require further research. Modeling the intraocular heat processes Modeling can be used as an alternative to conventional methods of temperature assessment inside a biological system that cannot be investigated directly, and the human eye is such a system. Modeling is the best tool for predicting the intraocular processes influenced by medical techniques that are used in clinical ophthalmology and change the heat balance in the eye. Early theoretical models of heat transfer in the eye in humans and in animals were developed to investigate thermal effects of microwave radiation on ocular structures. The results obtained by these models were verified by experimental measurements of intraocular heat processes under hyperthermic conditions and determining thermal characteristics of some intraocular structures.[14, 96, 97]. A mathematical model has been developed by Lagendijk [98] to calculate transient and steady state temperature distributions in normal unexposed human and rabbit eyes, and human and rabbit eyes heated by various heating techniques. The thermal conductivity and the specific heat of the lens of the rabbit eye were determined empirically. The heat transfer coefficients obtained were subsequently used by others in their studies. Scott [94] developed a finite element model of heat transport in the human eye, calculated the intraocular temperature distribution, and assessed the effects of various factors on this distribution. The effects of the ambient temperature, body-core temperature, choroidal circulation, and evaporation and convection from the corneal surface on the temperature distribution in the human eye were considered. In addition, the effect of lens thermal conductivity on the temperature distribution in the anterior segment of the eye was determined.[94]. Others also reported their models for the effects of circulation on the temperature distribution in the human eye and orbit.[99] Ng and Ooi [100-102] developed 2-dimension (D) and 3D finite element models of the human eye to simulate its thermal steady state conditions based on the properties and parameters reported in the open literatures and conducted a study to compare the two models. Blood temperature, ambient temperature, and evaporation rate were found to be the major parameters affecting the ocular surface temperature. These authors also assessed the effects of aqueous humor hydrodynamics on heat transfer in the human eye.[100-102]. Rafiq and Khanday [103] believed blood perfusion, evaporation and ambient temperature to be the major factors influencing the temperature distribution in the eye. Gokul and colleagues [104] developed a finite element model of the human eye to investigate the thermal effects of eyelid closure and opening in human eye. They demonstrated that the blood flow in the choroid increases OST when the lids are closed, and facilitates maintaining a constant OST. Modelling has been successfully used to predict thermal phenomena in ocular tissues during various medical procedures like exposing retinal, corneal and ciliary body tissues to laser [105-108], epibulbar tumor cryosurgery [82], and implanting intraocular electronic devices. [109, 110]. Conclusion Therefore, although numerous relevant studies have been conducted, temperature distribution patterns of heat exchange processes in the human eye have not been yet sufficiently investigated. Temperature measurements alone should not suffice for further comprehensive research of the heat exchange in the eye. Advanced methods of heat flux registration and heat process modeling should be more actively employed. The combined application of means for measuring the temperature and heat flux density will allow obtaining valuable data on the processes of heat release from the ocular surface depending on the blood flow in the eye, intraocular pressure, and stage of the pathological process, and relevant changes with treatment. This, in turn, will improve our knowledge in the field of physiology of the eye, and will allow using the data obtained for developing novel advanced techniques for eye disease diagnosis and treatment.
References 1.Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Amsterdam: Elsevier Saunders; 2006. 890 p. 2.Freeman RD, Fatt I. Environmental influences on ocular temperature. Invest Ophthalmol. 1973;12(8):596-602. 3.Mayer SA, Sessler VA. Therapeutic Hypothermia. New York: Marcel Dekker; 2005. 402 p. 4.Kiyatkin EA. Brain temperature and its role in physiology and pathophysiology: Lessons from 20 years of thermorecording. Temperature (Austin). 2019;6(4):271-333. 5.Tsariov А. Target temperature management in clinical practice of intensive care for critical states. Emergency Medicine. 2014;7(62):186-191. 6.Avetisov SE, Novikov IA, Lutsevich EE, Reyn ES. Use of infrared thermography in ophthalmology. Vestn Oftalmol. 2017;133(6):99 105. 8.Martin DK, Fatt I. The presence of a contact lens induces a very small increase in the anterior corneal surface temperature. Acta Ophthalmol (Copenh). 1986;64(5):512-518. 9.Kudinov VA, Kartashov EM, Stefanyuk EV. Technical thermodynamics and heat transfer. Textbook for Academic Baccalaureate. Мoscow: Yurait; 2019. 454 p. 10.Savvin VN, Korotkova OL, Shishkin GP. The use of thermodynamic approaches in assessing the state of a living system. Vyatka Medical Bulletin. 2017; 2:40-44. 11.Lucia U. Bioengineering thermodynamics of biological cells. Theor Biol Med Model. 2015;12:29. 12.Grischenko TG, Dekusha LV, Vorobiov LY. Heat flow measuring: theory, metrology, practice. Book 1. Methods and means of heat flow measuring. Kiev: Institute of Engineering Thermophysics of NASU; 2017. 438 p. 13.Mapstone R. Determinants of corneal temperature. Br J Ophthalmol. 1968;52(10):729-41. 14.Purslow C, Wolffsohn J. The relation between physical properties of the anterior eye and ocular surface temperature. Optom Vis Sci. 2007;84(3):197-201. 15.Emery AF, Kramar P, Guy AW, Lin, JC. Microwave induced temperature rises in rabbit eyes in cataract research. J Heat Transfer. 1975;97(1):123-128. 16.Holmberg A. The temperature of the eye during the application of hot packs, and after milk injections. Acta Ophthalmol (Copenh). 1952;30(4):348-364. 17.Zeiss E. Über Wärmestrahlungsmessungen an der lebenden menschlichen Hornhaut. Arch Augenheilkd. 1930;102:523–550. 18.Mapstone R. Measurement of corneal temperature. Exp Eye Res. 1968;7(2):237-43. 19.Purslow C, Wolffsohn JS. Ocular surface temperature: a review. Eye Contact Lens. 2005;31(3):117-123. 20.Buiko AS, Tsyikalo AL, Terenteva LS. Liquid crystal thermography in oncoophthalmology. J Ophthalmol (Ukraine). 1977;2:110-114. 21.Guo S, Wu K, Li C, Wang H, Sun Z, Xi D, Zhang S, Ding W, Zaghloul ME, Wang C, Castro FA, Yang D, Zhao Y. Integrated contact lens sensor system based on multifunctional ultrathin MoS2 transistors. Matter. 2021;4(3):969-985. 22.Moreddu R, Elsherif M, Butt H, Vigolo D, Yetisen AK. Contact lenses for continuous corneal temperature monitoring. RSC Adv. 2019;9(20):11433-11442. 23.Chang TC, Hsiao YL, Liao SL. Application of digital infrared thermal imaging in determining inflammatory state and follow-up effect of methylprednisolone pulse therapy in patients with Graves’ ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246(1):45-9. 24.Kawasaki S, Mizoue S, Yamaguchi M, Shiraishi A, Zheng X, Hayashi Y, Ohashi Y. Evaluation of filtering bleb function by thermography. Br J Ophthalmol. 2009;93(10):1331-6. 25.Wang C, Jiao H, Anatychuk L, Pasyechnikova N, Naumenko V, Zadorozhnyy O, Vikhor L, Kobylianskyi R, Fedoriv R, Kochan O. Development of a Temperature and Heat Flux Measurement System Based on Microcontroller and its Application in Ophthalmology. Measurement Science Review. 2022;22(2):73-79. 26.Anatychuk L, Pasyechnikova N, Zadorozhnyy O, Nazaretian R, Myrnenko V, Kobylyanskyi R, Gavrilyuk N. Original device and approaches to the study of temperature distribution in various eye segments (experimental study). J Ophthalmol (Ukraine). 2015;6:50-53. 27.Schwartz B, Feller MR. Temperature gradients in the rabbit eye. Invest Ophthalmol. 1962;1:513-21. 28.Nazaretian RE, Zadorozhnyy OS, Umanets NN, Naumenko VA, Pasyechnikova NV, Shafranskii VV. Intraocular temperature changes during vitrectomy procedure. J Ophthalmol (Ukraine). 2018;6:30-4. 29.Iguchi Y, Asami T, Ueno S, Ushida H, Maruko R, Oiwa K, Terasaki H. Changes in vitreous temperature during intravitreal surgery. Invest Ophthalmol. 2014;55(4):2344-9. 30.Mansouri K, Gillmann K, Rao HL, Szurman P, Weinreb RN; ARGOS -2 Study Group. Measurement of intraocular temperature in glaucoma: week-day and seasonal fluctuations. Br J Ophthalmol. 2022;bjophthalmol-2021-320495. 31.Horven I. Corneal temperature in normal subjects and arterial occlusive disease. Acta Ophthalmol (Copenh). 1975;53(6):863-874. 32.Alio` J, Padron M. Influence of age on the temperature of the anterior segment of the eye: measurements by infrared thermometry. Ophthalmic Res. 1982;14:153-159. 33.Martin DK, Fatt I. The presence of a contact lens induces a very small increase in the anterior corneal surface temperature. Acta Ophthalmol (Copenh). 1986;64(5):512-518. 34.Koçak I, Orgül S, Flammer J. Variability in the measurement of corneal temperature using a noncontact infrared thermometer. Ophthalmologica. 1999;213(6):345-349. 35.Morgan PB, Soh MP, Efron N, Tullo AB. Potential Applications of Ocular Thermography. Optom Vis Sci. 1993;70(7):568-76. 36.Craig JP, Singh I, Tomlinson A, Morgan PB, Efron N. The role of tear physiology in ocular surface temperature. Eye (Lond). 2000;14(4):635-641. 37.Tan L, Cai ZQ, Lai NS. Accuracy and sensitivity of the dynamic ocular thermography and inter-subjects ocular surface temperature (OST) in Chinese young adults. Cont Lens Anterior Eye. 2009;32(2):78-83. 38.Kamao T, Yamaguchi M, Kawasaki S, Mizoue S, Shiraishi A, Ohashi Y. Screening for dry eye with newly developed ocular surface thermographer. Am J Ophthalmol. 2011;151(5):782-791.e1. 39.Sodi A, Matteoli S, Giacomelli G, Finocchio L, Corvi A, Menchini U. Ocular surface temperature in age-related macular degeneration. J Ophthalmol. 2014;2014:281010.Crossref PubMed 40.Abreau K, Callan C, Kottaiyan R, Zhang A, Yoon G, Aquavella JV, Zavislan J, Hindman HB. Temperatures of the ocular surface, lid, and periorbital regions of sjögren's, evaporative, and aqueous-deficient dry eyes relative to normals. Ocul Surf. 2016;14(1):64-73. 41.Anatychuk LI, Pasyechnikova NV, Naumenko VА, Zadorozhnyy OS, Gavrilyuk MV, Kobylianskyi RR. A thermoelectric device for ophthalmic heat flux density measurements: results of piloting in healthy individuals. J Ophthalmol (Ukraine). 2019; 3:45-51. 42.Matteoli S, Vannetti F, Sodi A, Corvi A. Infrared thermographic investigation on the ocular surface temperature of normal subjects. Physiol Meas. 2020;41(4):045003. 43.Chandrasekar B, Rao AP, Murugesan M, Subramanian S, Sharath D, Manoharan U, Prodip B, Balasubramaniam V. Ocular surface temperature measurement in diabetic retinopathy. Exp Eye Res. 2021;;211:108749. 44.Mapstone R. Ocular thermography. Br J Ophthalmol. 1970;54(11):751-4. 45.Haber-Olguin A, Polania-Baron EJ, Trujillo-Trujillo F, Graue Hernandez EO. Thermographic behaviour of the cornea during treatment with two excimer laser platforms. Transl Vis Sci Technol. 2021;10(9):27. 46.Purslow C, Wolffsohn JS, Santodomingo-Rubido J. The effect of contact lens wear on dynamic ocular surface temperature. Cont Lens Anterior Eye. 2005;28(1):29-36. 47.Tan JH, Ng EYK, Acharya UR, Chee C. Infrared thermography on ocular surface temperature: A review. Infrared Phys Techn. 2009;52:97-108. 48.Rysä P, Sarvaranta J. Corneal temperature in man and rabbit. Observations made using an infra-red camera and a cold chamber. Acta Ophthalmol (Copenh). 1974;52(6):810-6. 49.Petznick A, Tan JH, Boo SK, Lee SY, Acharya UR, Tong L. Repeatability of a new method for measuring tear evaporation rates. Optom Vis Sci. 2013;90(4):366-371. 50.Shah AM, Galor A. Impact of Ocular Surface Temperature on Tear Characteristics: Current Insights. Clin Optom (Auckl). 2021;13:51-62. 51.Morgan PB, Tullo A, Efron N. Infrared thermography of the tear film in dry eye. Eye (Lond). 1995;9:615-618. 52.Tan LL, Sanjay S, Morgan PB. Screening for dry eye disease using infrared ocular thermography. Cont Lens Anterior Eye. 2016;39(6):442-449. 53.Matteoli S, Favuzza E, Mazzantini L, Aragona P, Cappelli S, Corvi A, Mencucci R. Ocular surface temperature in patients with evaporative and aqueous-deficient dry eyes: a thermographic approach. Physiol Meas. 2017;38(8):1503-1512. 54.García-Porta N, Gantes-Nuñez FJ, Tabernero J, Pardhan S. Characterization of the ocular surface temperature dynamics in glaucoma subjects using long-wave infrared thermal imaging. J Opt Soc Am A Opt Image Sci Vis. 2019;36(6):1015-1021. 55.Giannetto C, Di Pietro S, Falcone A, Pennisi M, Giudice E, Piccione G, Acri G. Thermographic ocular temperature correlated with rectal temperature in cats. J Therm Biol. 2021;102:103104. 56.Dorokhova O, Zborovska O, Meng G, Zadorozhnyy O. Temperature of the ocular surface in the projection of the ciliary body in rabbits. J Ophthalmol (Ukraine). 2020;2(493):65-69.Crossref 57.Refinetti R. Circadian rhythmicity of body temperature and metabolism. Temperature. 2020;7(4):321-362. 58.Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530(3):565-574. 59.Morgan PB, Soh MP, Efron N. Corneal surface temperature decreases with age. Cont Lens Anterior Eye. 1999;22(1):11-13. 60.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147(5):801-10. 61.Anatychuk L, Pasyechnikova N, Naumenko V, Kobylianskyi R, Zadorozhnyy O. Temperature and heat flux density of the eye surface in healthy individuals with different subfoveal thickness of the choroid. Acta Ophthalmol. 2022;100: S267. 62.Sigler EJ, Randolph JC. Comparison of macular choroidal thickness among patients older than age 65 with early atrophic age-related macular degeneration and normals. Invest Ophthalmol. 2013;54(9):6307-13. 63.Anatychuk LI, Pasyechnikova NV, Naumenko VА, Zadorozhnyy OS, Hramenko NI, Kobylianskyi RR. Temperature of and heat flux density from the external ocular surface in diabetic retinopathy patients: a pilot study. J Ophthalmol (Ukraine). 2019;6:3-6. 64.Sudhalkar A, Chhablani JK, Venkata A, Raman R, Rao PS, Jonnadula GB. Choroidal thickness in diabetic patients of Indian ethnicity. Indian J Ophthalmol. 2015;63(12):912-6. 65.Gugleta K, Orgül S, Flammer J. Is corneal temperature correlated with blood-flow velocity in the ophthalmic artery? Curr Eye Res. 1999;19(6):496-501. 66.Galassi F, Giambene B, Corvi A, Falaschi G. Evaluation of ocular surface temperature and retrobulbar haemodynamics by infrared thermography and colour Doppler imaging in patients with glaucoma. Br. J. Ophthalmol. 2007;91:878–881. 67.Morgan PB, Smyth JV, Tullo AB, Efron N. Ocular temperature in carotid artery stenosis. Optom Vis Sci. 1999;76(12):850-4. 68.Sodi A, Giambene B, Falaschi G, Caputo R, Innocenti B, Corvi A, Menchini U. Ocular surface temperature in central retinal vein occlusion: preliminary data. Eur J Ophthalmol. 2007;17(5):755-9. 69.Blomqvist A, Engblom D. Neural mechanisms of inflammation-induced fever. Neuroscientist. 2018;24(4):381-399. 70.Efron N, Brennan NA, Hore J, Rieper K. Temperature of the hyperemic bulbar conjunctiva. Curr Eye Res. 1988;7(6):615-618. 71.Klamann MK, Maier AK, Gonnermann J, Klein JP, Bertelmann E, Pleyer U. Ocular surface temperature gradient is increased in eyes with bacterial corneal ulcers. Ophthalmic Res. 2013;49(1):52-6. 72.Mapstone R. Corneal thermal patterns in anterior uveitis. Br J Ophthalmol. 1968;52(12):917-921. 73.Kawali AA. Thermography in ocular inflammation. Indian J Radiol Imaging. 2013;23(3):281-3. 74.Leshno A, Stern O, Barkana Y, Kapelushnik N, Singer R, Prat DL, Cohen G, Ben-David G, Abrahami D, Huna-Baron R, Skaat A. Ocular surface temperature differences in glaucoma. Eur J Ophthalmol. 2022;32(3):1518-1524. 75.Zadorozhnyy OS, Guzun OV, Bratishko AIu, Kustrin TB, Nasinnik IO, Korol AR Infrared thermography of external ocular surface in patients with absolute glaucoma in transscleral cyclophotocoagulation: a pilot study. J Ophthalmol (Ukraine). 2018;2:23-28. 76.Zadorozhnyy OS, Guzun OV, Kustrin TB, Korol AR, Naumenko VA, Pasyechnikova NV. Ocular heat exchange indices in terminal neovascular glaucoma patients with proliferative diabetic retinopathy. J Ophthalmol (Ukraine). 2020;1:10-13. 77.Auker CR, Parver LM, Doyle T, Carpenter DO. Choroidal blood flow. I. Ocular tissue temperature as a measure of flow. Arch Ophthalmol. 1982;100(8):1323-6. 78.Konieczka K, Koch S, Hauenstein D, Chackathayil TN, Binggeli T, Schoetzau A, Flammer J. Effects of the Glaucoma Drugs Latanoprost and Brimonidine on Corneal Temperature. Transl Vis Sci Technol. 2019;8(3):47. 79.Merté HJ, Schubert E. Thermographische Untersuchungen. Albrecht von Graefes Arch Klin Ophthalmol. 1971;183:47-52. 80.Galassi F, Giambene B, Corvi A, Falaschi G, Menchini U. Retrobulbar hemodynamics and corneal surface temperature in glaucoma surgery. Int Ophthalmol. 2008;28(6):399-405. 81.Fujishima H, Toda I, Yagi Y, Tsubota K. Quantitative evaluation of postsurgical inflammation by infrared radiation thermometer and laser flare-cell meter. J Cataract Refract Surg. 1994;20(4):451-4. 82.Anatychuk L, Pasyechnikova N, Naumenko V, Kobylianskyi R, Nazaretyan R, Zadorozhnyy O. Prospects of Temperature Management in Vitreoretinal Surgery. Ther Hypothermia Temp Manag. 2021;11(2):117-121. 83.Zadorozhnyy OS, Savin NV, Buiko AS. Improving the technique for controlled cryogenic destruction of conjunctival tumors located in the projection of the ciliary body onto the sclera: A preliminary report. J Ophthalmol (Ukraine). 2018;5:60-65. 84.Betney S, Morgan PB, Doyle SJ, Efron N. Corneal temperature changes during photorefractive keratectomy. Cornea. 1997;16(2):158-61. 85.Maldonado-Codina C, Morgan PB, Efron N. Thermal consequences of photorefractive keratectomy. Cornea. 2001;20(5):509-515. 86.Haber-Olguin A, Polania-Baron EJ, Trujillo-Trujillo F, Graue Hernandez EO. Thermographic Behavior of the Cornea During Treatment With Two Excimer Laser Platforms. Transl Vis Sci Technol. 2021;10(9):27. 87.Sniegowski MC, Erlanger M, Olson J. Thermal imaging of corneal transplant rejection. Int Ophthalmol. 2018;38(6):2335-2339. 88.May DR, Freedland RJ, Charles S, Wang C, Bakos J. Ocular hypothermia: anterior chamber perfusion. Br J Ophthalmol. 1983;67(12):808-13. 89.Schwartz B. Environmental temperature and the ocular temperature gradient. Arch Ophthalmol. 1965;74:237-43. 90.Anatychuk L, Pasyechnikova N, Zadorozhnyy O, Kobylianskyi R, Nazaretyan R, Myrnenko V. Experimental study of intraocular temperature distribution in the rabbit under various environmental conditions. Acta Ophthalmol. 2016;94:S256. 91.Horiguchi M, Miyake Y. Effect of temperature on electroretinograph readings during closed vitrectomy in humans. Arch Ophthalmol. 1991;109(8):1127-1129. 92.Landers MB 3rd, Watson JS, Ulrich JN, Quiroz-Mercado H. Determination of retinal and vitreous temperature in vitrectomy. Retina. 2012;32(1):172-6. 93.Romano MR, Vallejo-Garcia JL, Romano V, Angi M, Vinciguerra P, Costagliola C. Thermodynamics of vitreoretinal surgery. Curr Eye Res. 2013;38(3):371-4. 94.Shinoda K, Matsumoto SC, Yagura K, Terauchi G, Shoji T, Yoshikawa Y, Igawa Y, Mizota A, Miyake Y. Intraocular Temperature Distribution in Eyes Undergoing Different Types of Surgical Procedures during Vitreous Surgery. J Clin Med. 2022;11(7):2053. 95.Scott JA. A finite element model of heat transport in the human eye. Phys Med Biol. 1988;33(2):227-41. 96.Buck B, Lopezcarasa G, Kon Jara VA, Mwanza J, Landers M. Retinal and intravitreal temperature during vitreous surgery. Invest Ophthalmol. 2014;55(13):1932. 97.Taflove A, Brodwin ME. Computation of the electromagnetic fields and induced temperatures within a model of the microwave-irradiated human eye. IEEE Transactions on Microwave Theory and Techniques. 1975;23(11):888-896, 98.Neelakantaswamy PS, Ramakrishnan KP, Microwave-induced hazardous nonlinear thermoelastic vibrations of the ocular lens in the human eye. Journal of Biomechanics. 1979;12(3):205-210. 99.Lagendijk JJ. A mathematical model to calculate temperature distributions in human and rabbit eyes during hyperthermic treatment. Phys Med Biol. 1982;27(11):1301-11. 100.Flyckt VM, Raaymakers BW, Lagendijk JJ. Modelling the impact of blood flow on the temperature distribution in the human eye and the orbit: fixed heat transfer coefficients versus the Pennes bioheat model versus discrete blood vessels. Phys Med Biol. 2006;51(19):5007-5021. 101.Ng EY, Ooi EH. FEM simulation of the eye structure with bio-heat analysis. Comput Methods Programs Biomed. 2006;82(3):268-76. 102.Ng EY, Ooi EH, Archarya UR. A comparative study between the two-dimensional and three-dimensional human eye models. Math. Comput Model. 2008;48:712–720. 103.Ooi EH, Ng EY. Simulation of aqueous humor hydrodynamics in human eye heat transfer. Comput Biol Med. 2008;38(2):252-62. 104.Rafiq A, Khanday MA. Thermal behavior of human eye in relation with change in blood perfusion, porosity, evaporation and ambient temperature. J Therm Biol. 2016;62:138-142. 105.Gokul KC, Gurung DB, Adhikary PR. Thermal effects of eyelid in human eye temperature model. Journal of Applied Mathematics & Informatics. 2014;32(5-6):649-663. 106.Narasimhan A, Jha KK. Bio-heat transfer simulation of retinal laser irradiation. Int J Numer Method Biomed Eng. 2012;28(5):547-59. 107.Truong LTD, Lesniewski PJ, Wedding AB. Heat transfer simulation in laser irradiated retinal tissues. Biomed Phys Eng Express. 2021;8(1). 108.Ooi EH, Ang WT, Ng EY. A boundary element model of the human eye undergoing laser thermokeratoplasty. Computers in Biology and Medicine. 2008;38(6):727-737. 109.Regal S, Troughton J, Delattre R, Djenizian T, Ramuz M. Changes in temperature inside an optomechanical model of the human eye during emulated transscleral cyclophotocoagulation. Biomed Opt Express. 2020;11(8):4548-4559. 110.Gongal D, Thakur S, Panse A, Pawar R, Yu CQ, Foster CD. Thermal analysis of intraocular electronic display projector visual prosthesis. Numeri Heat Transf A Appl. 2020;78(12):706-716. 111.Opie NL, Burkitt AN, Meffin H, Grayden DB. Heating of the eye by a retinal prosthesis: modeling, cadaver and in vivo study. IEEE Trans Biomed Eng. 2012;59(2):339-45. Disclosures Author Contributions: The authors confirm the following contributions to the article—study conception and design: NP; data collection and analysis: OZ, AK and VN; drafting of the manuscript: OZ. All authors read and approved the final manuscript. Funding sources: The study is a part of the institute’s experimental and clinical research program entitled Heat Exchange in the Eye Depending on the Morphological and Functional State of the Choroid and After Vitreoretinal and Glaucoma Surgery (state registration № 0117U004356). Conflict of interest: All authors have read the journal’s Author Agreement and Conflict of Interest policy. The authors have no potential conflict of interest to declare. Abbreviations: DR, diabetic retinopathy; HF, heat flux; IOP, intraocular pressure; IOT, intraocular temperature; IR, infrared; OST, ocular surface temperature
|