J.ophthalmol.(Ukraine).2022;6:10-13.
|
http://doi.org/10.31288/oftalmolzh202261013 Received: 07.09.2022; Accepted: 04.11.2022; Published on-line: 21.12.2022 Our experience of laser iridotomy in patients with chronic angle closure glaucoma R. M. Lopadchak, I. Ia. Novytskyy, Ya. Z. Fedus Danylo Halytsky Lviv National Medical University Lviv (Ukraine) TO CITE THIS ARTICLE: Lopadchak RM, Novytskyy IIa, Fedus YaZ. Our experience of laser iridotomy in patients with chronic angle closure glaucoma. J.ophthalmol.(Ukraine).2022;6:10-13. http://doi.org/10.31288/oftalmolzh202261013
Background: Determining the strategy of surgical treatment for chronic angle closure glaucoma (CACG) is still important. Purpose: To assess the efficacy, rates of successful intraocular pressure (IOP) lowering and the increase in anterior chamber angle for laser peripheral iridotomy (LPI) in patients with CACG. Material and Methods: We examined 31 patients (31 eyes; 18 women and 13 men) who had undergone LPI for CACG. Patient age ranged from 49 to 77 years (mean age, 64.8 ± 5.3 years). The indication for LPI was an anterior chamber angle of Shaffer grade 2 to 3 and the presence of optic neuropathy. Mean Maklakoff intraocular pressure (IOP) was 23.4 ± 1.2 mmHg. Results: Thirteen patients required surgery during the follow-up after LPI. Particularly, 10 eyes required phacoemulsification (PHACO) only, and 3 eyes, PHACO plus goniosynechialysis (GSL), because of decompensated IOP and an anterior chamber angle narrower than Shaffer grade 2 by gonioscopy. Kaplan-Meier survival analysis showed that the rates of successful IOP lowering for laser peripheral iridotomy (without the need for surgery, PHACO alone or PHACO plus GSL) at 12 months, 24 months and 36 months were 87.1%, 71.0% and 58.06%, respectively. Conclusion: The LPI is effective for opening the anterior chamber angle and reducing IOP over 6 months, but is less effective and does not allow opening the anterior chamber angle over a period exceeding 6 months. The LPI may be considered as a preparatory procedure in CACG, particularly in the presence of an acute angle-closure glaucoma attack in the fellow eye. Keywords: chronic angle closure glaucoma, laser peripheral iridotomy, gonioscopy, phacoemulification, goniosynechialysis, anterior chamber angle optical coherence tomography
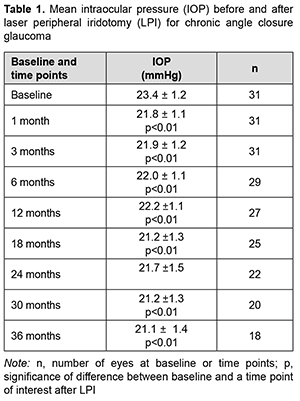
Introduction It was estimated that the number of people with angle-closure glaucoma in 2020 would rise from 21 million to 23.36 million for the world and 1.46 to 1.57 million for Europe [1, 2]. Determining the strategy of surgical treatment for chronic angle closure glaucoma (CACG) is still important, because trabeculectomy does not allow opening the anterior chamber angle (ACA), and at least 180º of iridotrabecular contact is seen in approximately 47% of patients that underwent laser peripheral iridotomy (LPI) [3]. According to the European Glaucoma Society Terminology and Guidelines for Glaucoma, the first phase of management for CACG consists of medical treatment plus LPI. However, the efficacy and survival of IOP lowering effect of LPI in patients with CACG have not been sufficiently studied. The purpose of the study was to assess the efficacy and survival of IOP lowering effect of and the increase in anterior chamber angle for laser peripheral iridotomy (LPI) in patients with CACG. Material and Methods We followed 31 patients (31 eyes; 18 women and 13 men) who had undergone LPI for chronic angle closure glaucoma. Patient age ranged from 49 to 77 years (mean age, 64.8 ± 5.3 years). Of these patients, 10 had emmetropia; 17, mild hyperopia (+0.5D to +2.0D), and 4, moderate hyperopia (+2.5D to +3.5D). Of the 31 fellow eyes of the patients involved in the study, 14 had chronic acute angle closure glaucoma; 7 had a history of acute of chronic acute angle closure glaucoma event; 4, terminal angle-closure glaucoma; and 6 were suspects for angle closure glaucoma. Of the 14 fellow eyes that had chronic acute angle closure glaucoma before the study, 6 underwent LPI; 3 underwent peripheral iridectomy; and 5 underwent cataract phacoemulsification (PHACO). In addition, of the 31 fellow eyes, 12 are on medical treatment. The indication for LPI was an anterior chamber angle of Shaffer grade 2 to 3 and the presence of optic neuropathy. Patients underwent a routine eye examination, gonioscopy, ACA optical coherence tomography (OCT), tonometry, and static perimetry preoperatively and at day 7 and months 1, 3, 6, 12, 18, 24, 30 and 36 after surgery. The number of glaucoma medications used was noted. The Nesterov’s simplified tonography was conducted, with a true IOP determined using with a 5-g weight. To perform tonometry, a 15.0-g weight was placed on the cornea for 4 minutes with the stained side down. Thereafter, IOP was assessed with a 15-g weight. The diameters of the imprints obtained were measured with a ruler, and tables were used to determine the coefficient of the aqueous outflow facility. The minimum follow-up period was 3 years. Laser iridotomy procedure: Preoperative topical anesthetic was instilled. A YAG-laser (ALCON LABORATORIES 3000LE Ophthalmic Laser; 1.4-1.8 mJ power) and Ocular® Wise iridotomy-sphincterotomy 103D magnifying argon/diode laser lens were used to perform iridotomy in the temporal quadrant at 3 o’clock and nasal quadrant at 9 o’clock at 2 mm from the iris root. Surgery efficacy was confirmed by the occurrence of iris coloboma, liberation of iris pigment, fundus reflex seen through the coloboma, and the hole seen on the OCT. Postoperatively, topical dexamethasone was administered three times daily for seven days, and oral acetozolamide 0.25 g (Diacarb, Polpharma, Starogard Gdanski; Poland), once daily for one day. Hypotensive medications were continued for 7 days after surgery and gradually tapered thereafter as the IOP and ACA allowed. Ethical approval was obtained from the Ethics Committee of the Lviv National Medical University and the study adhered to the tenets of the Declaration of Helsinki and the relevant laws of Ukraine. Microsoft Excel software was used for statistical analyses. Data are presented as mean and standard deviation (SD). Student's paired t-test was used to determine the significance of differences between dependent groups. The level of significance p ≤ 0.05 was assumed. Kaplan-Meier estimate was applied to assess the survival of IOP lowering effect of LPI in patients with CACG. The requirement for resurgery indicated the loss of efficacy of LPI. Results Surgery was unremarkable in all cases. IOP spikes, corneal edema and hyphema were seen in the early postoperative period in 8 patients 5 patients, and one patient, respectively. Mean IOP decreased from 23. 4 ± 1.2 mmHg at baseline to 21.8 ± 1.1 mmHg at one month after surgery (p < 0.01), and did not change substantially over the follow-up period (p < 0.01). At 36 months, mean IOP was 21.1 ± 1.4 mmHg (p < 0.01) (Table 1).
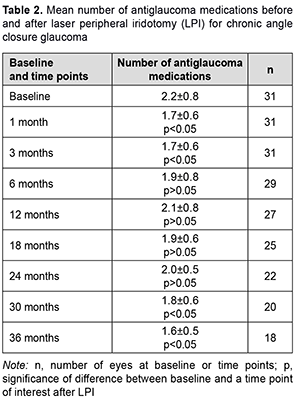
Mean number of medications decreased from 2.2 ± 0.8 at baseline to 1.7 ± 0.6 mmHg at one month after surgery (p < 0.05), but almost returned to baseline after 6 months, and practically did not change over the follow-up period (p > 0.05). At 36 months, mean number of medications was 1.6 ± 0.5 (p < 0.05) compared to 2.2 ± 0.8 at baseline (Table 2).
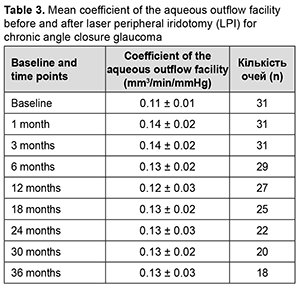
Mean coefficient of the aqueous outflow facility was 0.11 ± 0.01 mm3/min/mmHg at baseline, improved insignificantly to 0.14 ± 0.02 mm3/min/mmHg at 1 month (p > 0.05), and did not change substantially afterwards (Table 3).
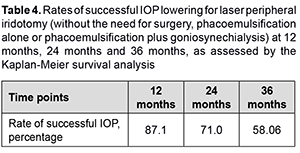
Mean width of anterior chamber angle determined by OCT was 26.2 ± 4.6о, and improved to 28.4 ± 3.9о (p < 0.05) at 1 month after LPI. Of the 31 study eyes, 5 (16.12%) required treatment with a single antiglaucoma medication; 10 (32.25%), fixed combination antiglaucoma therapy; and 13 (41.93%), surgical treatment, and only 3 (9.7%) did not require any treatment after LPI over the follow up. Thirteen patients required surgery from month 6 after LPI. Particularly, 10 eyes required PHACO only, and 3 eyes, PHACO plus goniosynechialysis (GSL), because of decompensated IOP and an anterior chamber angle narrower than Shaffer grade 2 by gonioscopy. The number of operated eyes was 4 (12.9%) by month 12, and increased to 9 (29%) by month 24 and to 13 (41.9%) by month 36. Kaplan-Meier survival analysis showed that the rates of successful IOP lowering for laser peripheral iridotomy (without the need for surgery, PHACO alone or PHACO plus GSL) at 12 months, 24 months and 36 months were 87.1%, 71.0% and 58.06%, respectively (Table 4).
Discussion Our findings are close to those reported by others. Thus, in a study by Rao and colleagues [4], of the 68 eyes with angle closure glaucoma which had undergone LPI during the study period, 16 (23.5%) required treatment with a single glaucoma medication; 19 (28%), fixed combination antiglaucoma therapy; and 24 (35.3%), surgical treatment, and only 9 (12%) did not require any treatment after LPI over a follow up of 50 ± 23 months. In a study by Chen and colleagues [2], of the 103 eyes of the CACG group which had undergone LPI, 21 (20.4%) received surgery, either trabeculectomy alone (13 eyes) or trabeculectomy combined with extracapsular cataract extraction and intraocular lens implantation (8 eyes) [5]. According to Alsagoff and colleagues [6] and Chew and colleagues [7], despite the presence of a patent LPI, most eyes with established PACG require further treatment to control IOP, and, medical therapy fails in most cases of chronic PACG, necessitating surgery (particularly, phacoemulsification). Conclusion First, laser peripheral iridotomy is effective for opening the anterior chamber angle and reducing IOP over 6 months. Second, laser peripheral iridotomy is less effective for reducing IOP and does not allow opening the anterior chamber angle over a period exceeding 6 months. Rates of successful IOP lowering effect for laser peripheral iridotomy at 12 months, 24 months and 36 months were 87.1%, 71.0% and 58.06%, respectively. Finally, laser peripheral iridotomy may be considered as a preparatory procedure in chronic angle closure glaucoma, particularly in the presence of an acute angle-closure glaucoma attack in the fellow eye.
References 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006 Mar;90(3):262-7. 2.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. 3.Ophthalmology. 2014 Nov;121(11):2081-90. 4.Peng PH, Nguyen H, Lin HS, et al. Long-term outcomes of laser iridotomy in Vietnamese patients with primary angle closure. Br J Ophthalmol. 2011;95(9):1207-1211. 5.Rao A, Rao HL, Kumar AU, Babu JG, Madhulata U, Arthi J, et al. Outcomes of Laser Peripheral Iridotomy in Angle Closure Disease. Semin Ophthalmol. 2013;28:4e8. 6.Chen MJ, Cheng CY, Chou CK, et al. The long-term effect of Nd:YAG laser iridotomy on intraocular pressure in Taiwanese eyes with primary angle-closure glaucoma. J Chin Med Assoc 2008;71:300–4. 7.Аlsagoff Z, Aung T, Ang LP, Chew PT. Long-term clinical course of primary angle-closure glaucoma in an Asian population. Ophthalmology. 2000;107:2300–4. 8.Chew PT, Aung T. Primary angle-closure glaucoma in Asia. J Glaucoma. 2001;10(suppl):S7– 8.
Disclosures Corresponding Author: Lopadchak R.M., e-mail: rlopadchak@yahoo.com Author Contributions: IN - concept, design, data analysis, drafting the manuscript. RL: concept, design, data collection and interpretation, research, drafting the manuscript. YaF: concept, design, research, data interpretation. All authors analyzed the results and agreed on the final version of the manuscript. Disclaimer: The authors declare that the opinions expressed in this article are their own and not the official positions of the institution. Conflict of interest: The authors have no conflicts of interest, including financial interests, activities, relationships, or affiliations. Sources of support: none. Ethical Statement: This research was conducted with the participation of people. All study participants signed informed consent. The permission of the local Bioethics Committee was obtained. The study was conducted in accordance with the Declaration of Helsinki. The study does not include experiments on animals. Abbreviations: CACG, chronic angle closure glaucoma; ACA, anterior chamber angle; IOP, intraocular pressure; LPI, laser peripheral iridotomy; PHACO, cataract phacoemulsification; GSL, goniosynechialysis; OCT, optical coherence tomography; PACG, primary angle-closure glaucoma; IOL, intraocular lens.
|